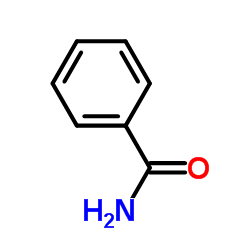
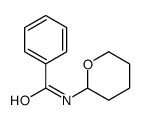
|
~% |
|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~64% |
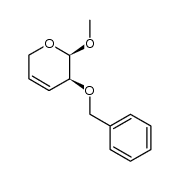
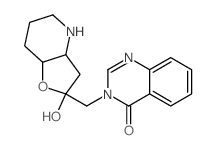
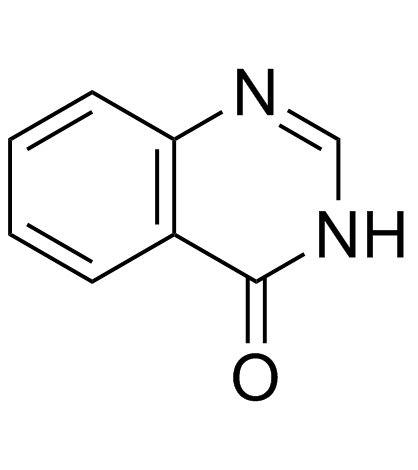
![2,3-cis-3-benzyloxy-1-benzyloxycarbonyl-2-[2-oxo-3-(4-oxo-4H-quinazolin-3-yl)propyl]piperidin Structure](https://image.chemsrc.com/caspic/202/391242-46-9.png)

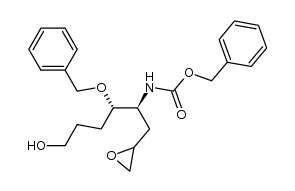
![benzyl {2-benzyloxy-5-hydroxy-1-[2-hydroxy-3-(4-oxo-4H-quinazolin-3-yl)propyl]-pentyl}carbamate Structure](https://image.chemsrc.com/caspic/116/391242-48-1.png)