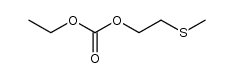
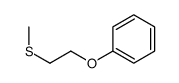
|
~99% |
|
~28% |
|
~38% |
|
~28% |
|
~71% |
|
~% |
|
~56% |
|
~% |
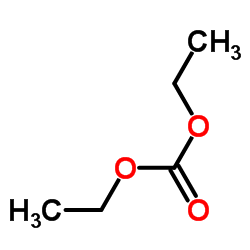

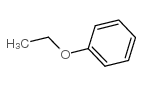

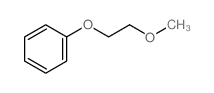

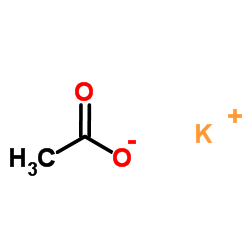
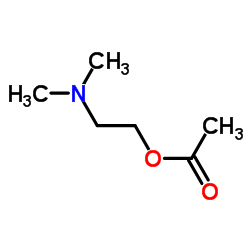

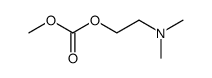
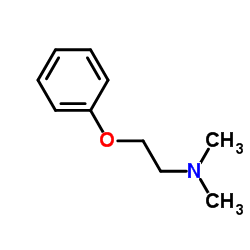

![Benzene,[(2-phenoxyethyl)thio]- Structure](https://image.chemsrc.com/caspic/492/17414-04-9.png)