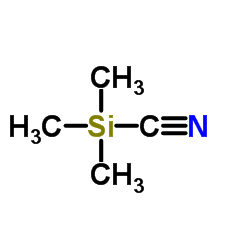
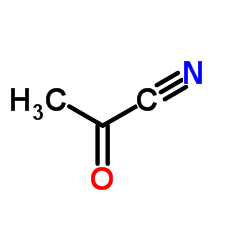
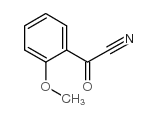
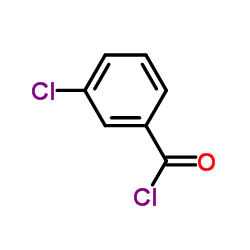
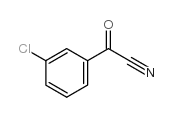
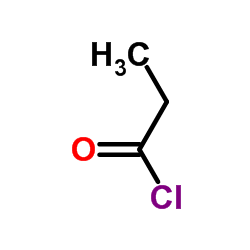
|
~90% |
|
~84% |
|
~94% |
|
~80% |