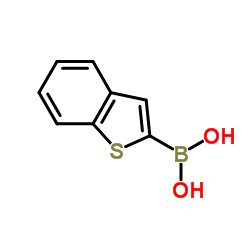
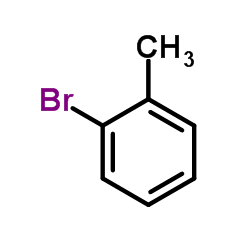
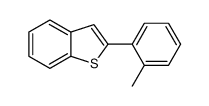
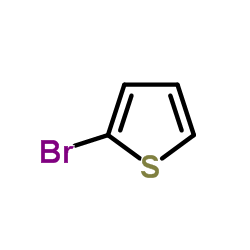
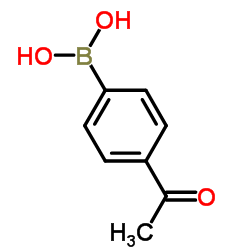
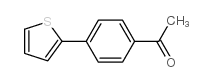
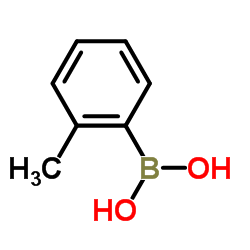
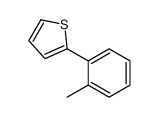
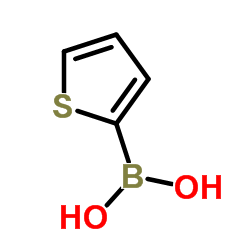
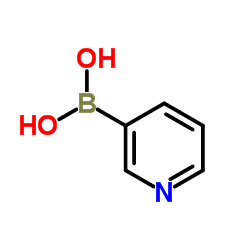
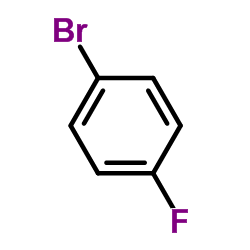
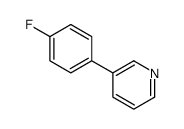
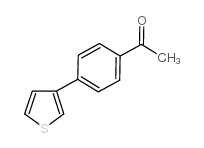
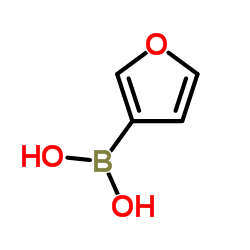
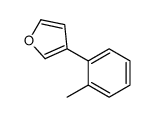
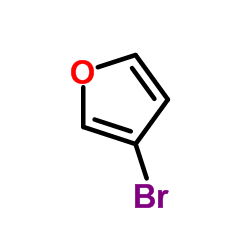
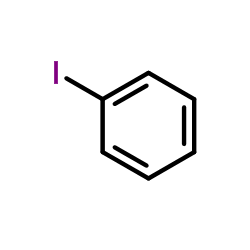
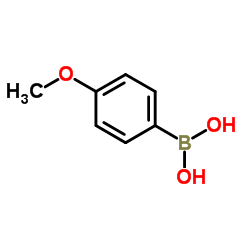
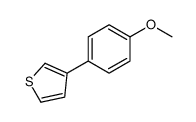
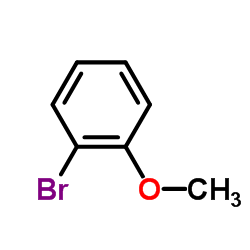
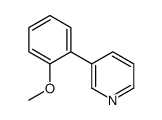
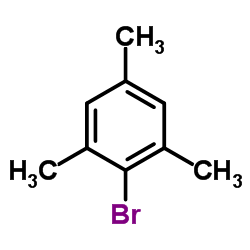
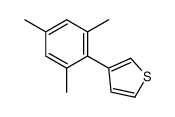
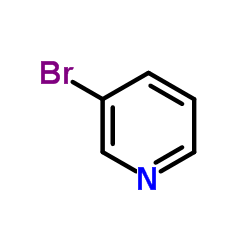
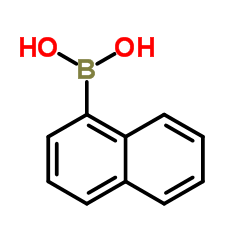
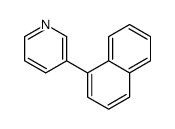
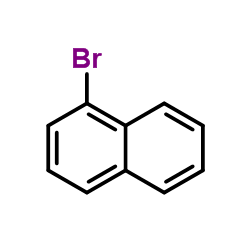
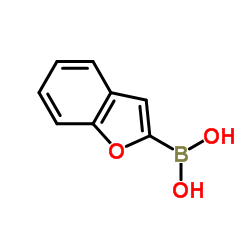
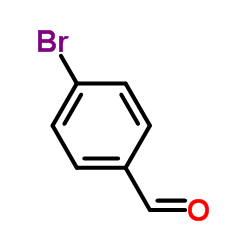
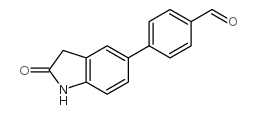
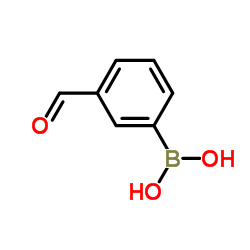
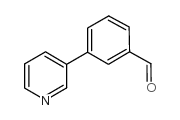
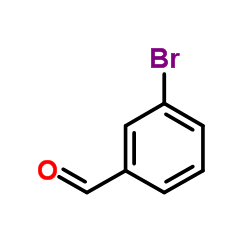
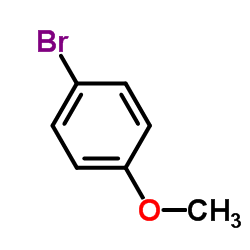
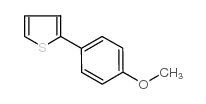
|
~92% |
|
~92% |
|
~87% |
|
~87% |
|
~81% |
|
~86% |
|
~79% |
|
~70% |
|
~80% |
|
~75% |
|
~61% |
|
~75% |
|
~79% |
|
~84% |
|
~94% |
|
~89% |
|
~86% |
|
~80% |