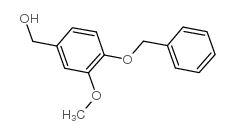
|
~92% |
|
~% |
|
~0% |
|
~99% |
|
~95% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
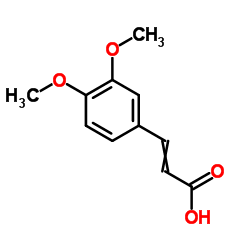
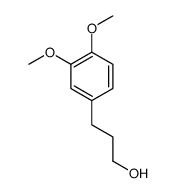
![3-BENZO[1,3]DIOXOL-5-YL-PROPAN-1-OL Structure](https://image.chemsrc.com/caspic/040/7031-03-0.png)
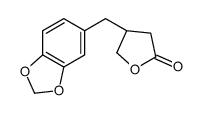



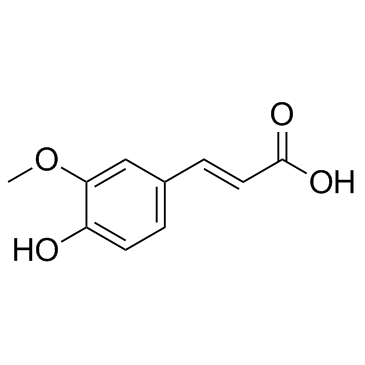
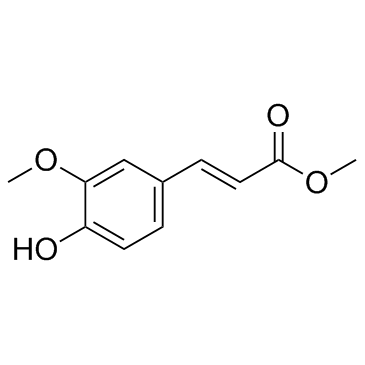
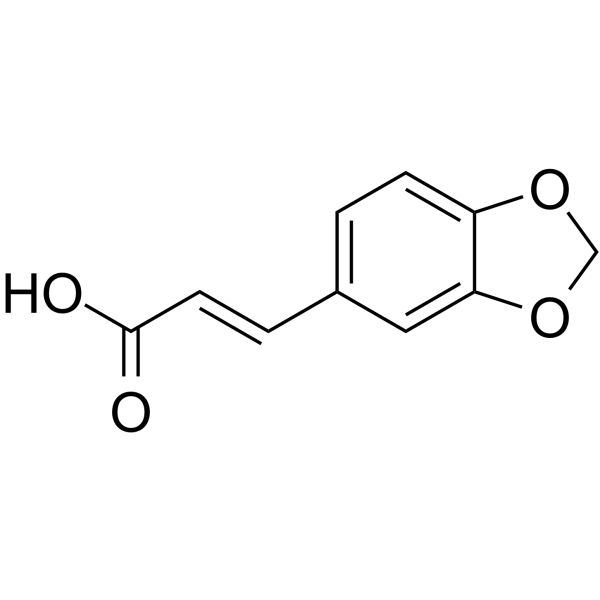

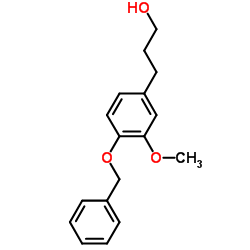
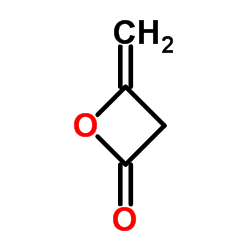
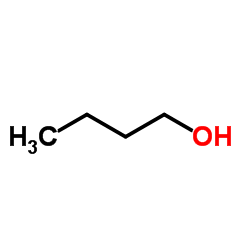
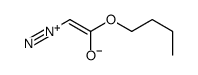
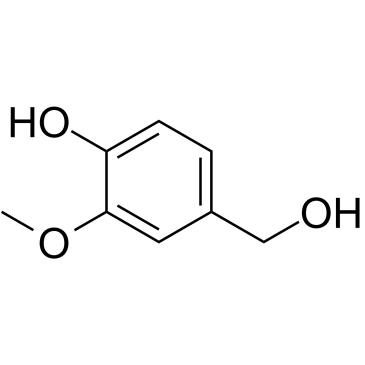
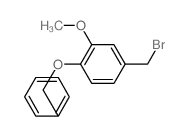


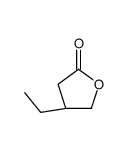
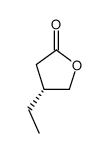

![(R)-4-[(3-methoxyphenyl)methyl]dihydro-2(3H)-furanone Structure](https://image.chemsrc.com/caspic/493/77756-19-5.png)
![(4S)-4-[(3-methoxyphenyl)methyl]oxolan-2-one Structure](https://image.chemsrc.com/caspic/058/171230-53-8.png)