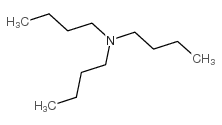
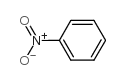
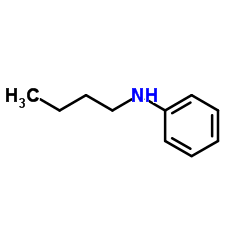
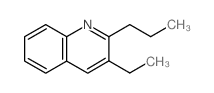
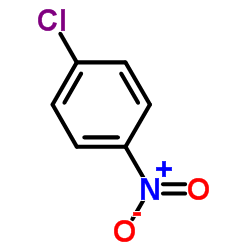
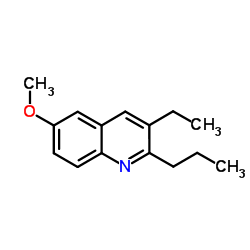
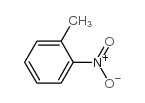
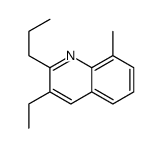
|
~56% |
|
~39% |
|
~41% |
|
~22% |
|
~58% |
|
~0% |