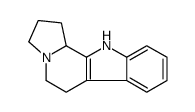
|
~94% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
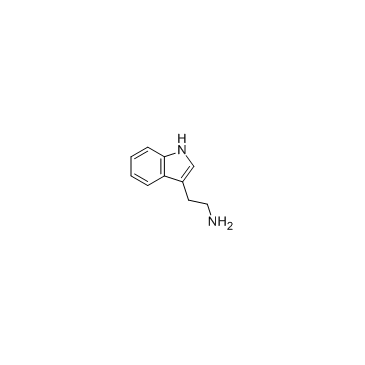
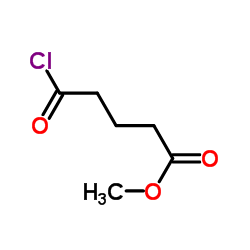
![Butanoic acid,4-[[2-(1H-indol-3-yl)ethyl]amino]-4-oxo-, methyl ester Structure](https://image.chemsrc.com/caspic/176/92870-51-4.png)
![1,2,5,6,11,11b-Hexahydro-3H-indolizino[8,7-b]indol-3-one Structure](https://image.chemsrc.com/caspic/423/32283-51-5.png)
![1,2,5,6,11,11b-hexahydro-indolizino[8,7-b]indole-3-thione Structure](https://image.chemsrc.com/caspic/022/59032-44-9.png)