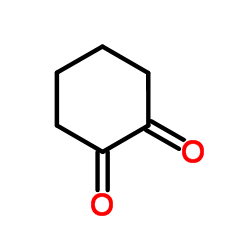
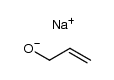
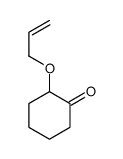
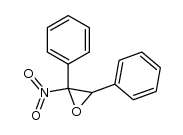
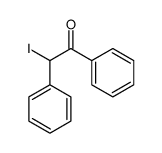
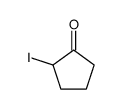
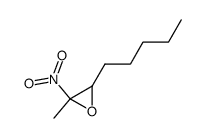
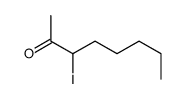
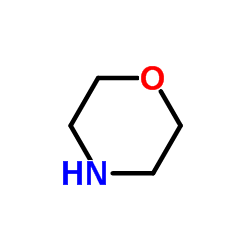
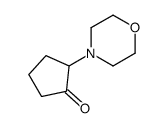
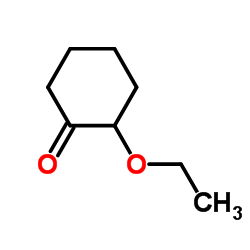
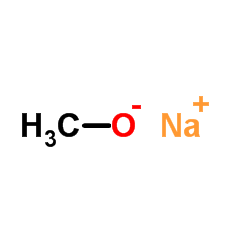
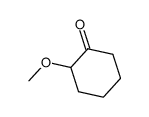
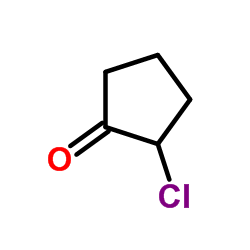
|
~75% |
|
~81% |
|
~40% |
|
~61% |
|
~% |
|
~92% |
|
~% |
|
~86% |
|
~78% |
|
~% |
|
~78% |
|
~% |
|
~72% |
|
~75% |
|
~% |
|
~62% |
|
~65% |
|
~% |
|
~82% |
|
~% |
|
~70% |

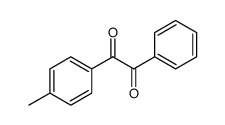
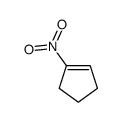
![1-nitro-6-oxabicyclo[3.1.0]hexane Structure](https://image.chemsrc.com/caspic/403/109314-64-9.png)