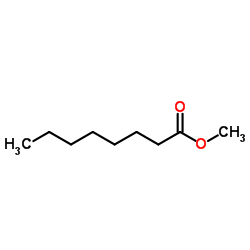
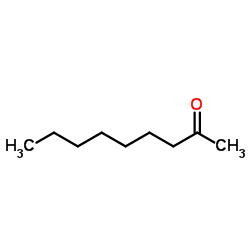
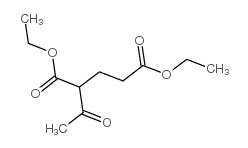
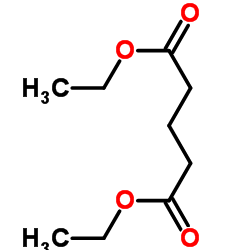
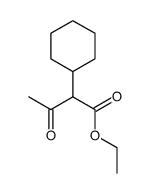
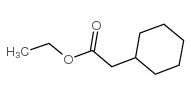
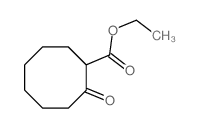
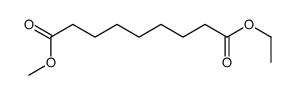
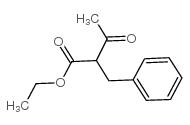
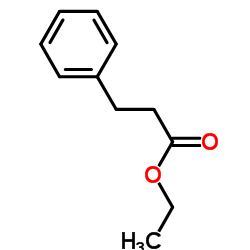
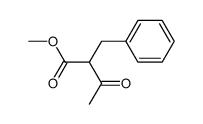
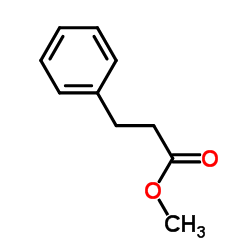
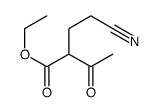
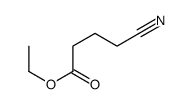
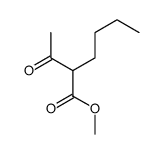
|
~86% |
|
~80% |
|
~92% |
|
~85% |
|
~92% |
|
~59% |
|
~94% |
|
~87% |
|
~51% |
|
~57% |
|
~87% |