|
~% |
|
~79% |
|
~% |
|
~% |
|
~10% |
|
~99% |
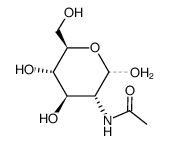
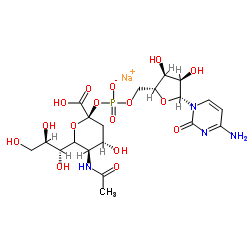
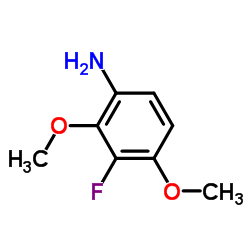
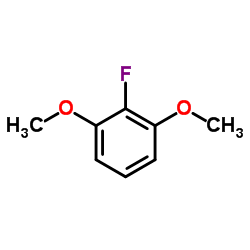
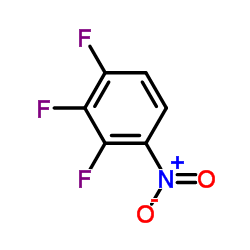
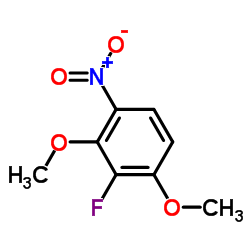
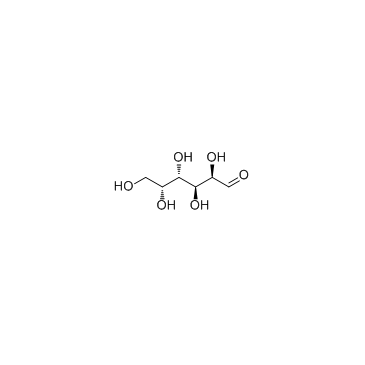

![(2,2,7,7-Tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-5-yl)methanol Structure](https://image.chemsrc.com/caspic/222/4064-06-6.png)