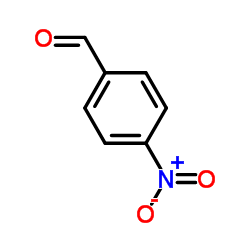
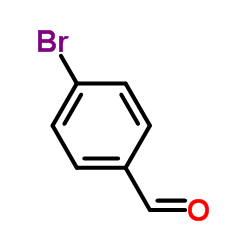
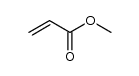
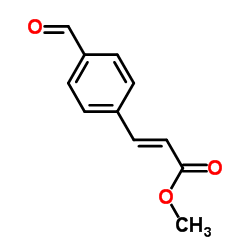
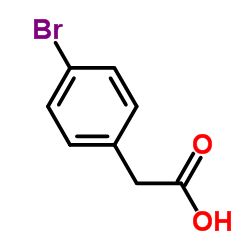
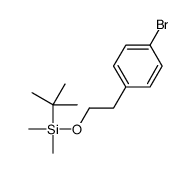
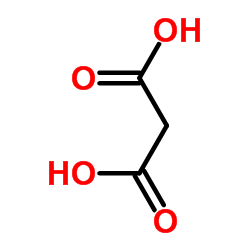
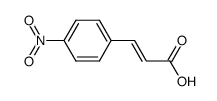
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~96% |
|
~98% |
|
~% |
|
~99% |

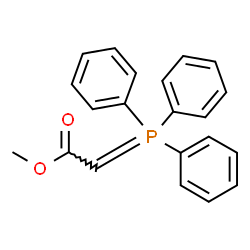
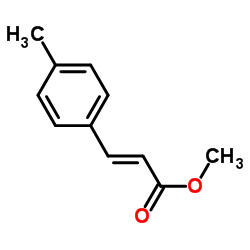
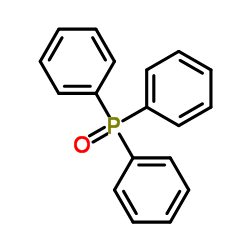

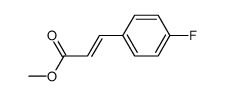

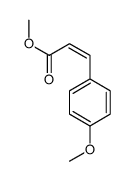
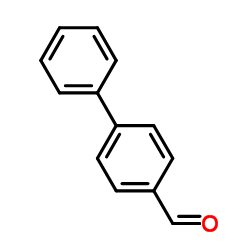
![(E)-methyl 3-([1,1’-biphenyl]-4-yl)acrylate Structure](https://image.chemsrc.com/caspic/325/20883-99-2.png)