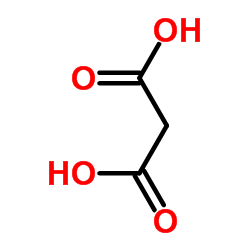
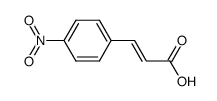
|
~0% |
|
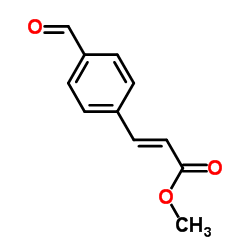
~0% |
|
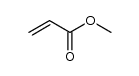
~0% |
|
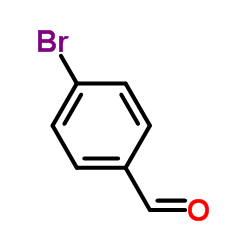
~0% |
|
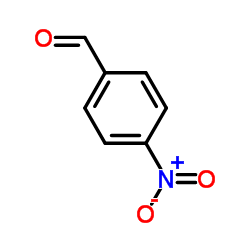
~0% |
|
~96% |
|
~98% |
|
~% |
|
~99% |

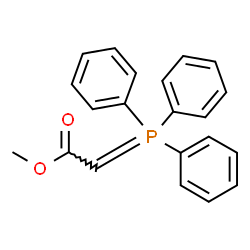
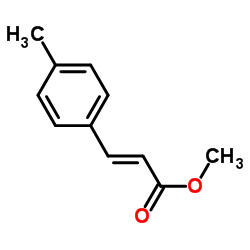
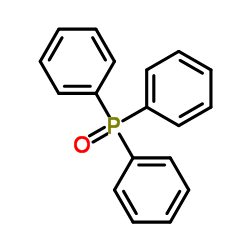

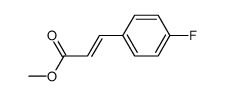

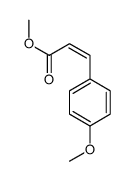
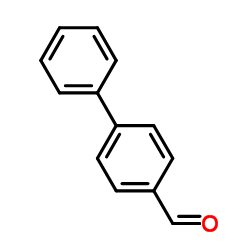
![(E)-methyl 3-([1,1’-biphenyl]-4-yl)acrylate结构式](https://image.chemsrc.com/caspic/325/20883-99-2.png)

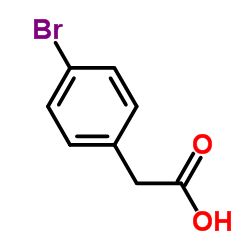
![1-溴-4-[2-(叔丁基二甲基甲硅烷基氧基)乙基]苯结构式](https://image.chemsrc.com/caspic/127/73899-15-7.png)