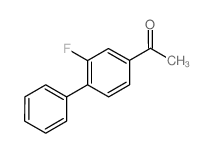
|
~90% |
|
~% |
|
~80% |
|
~95% |
|
~89% |
|
~80% |
|
~92% |
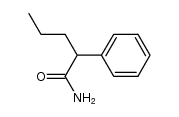
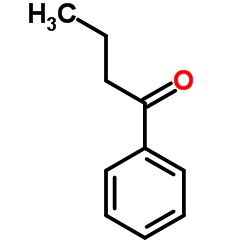
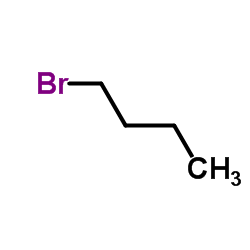
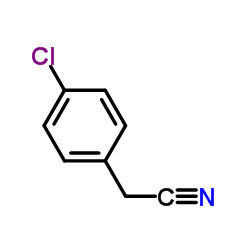
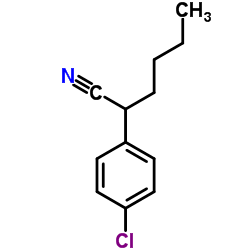
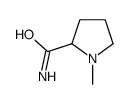
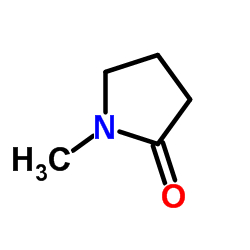
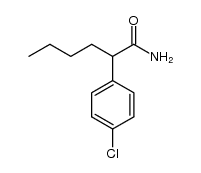
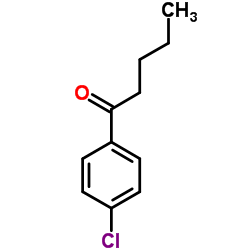
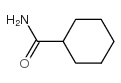

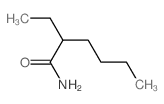
![2-(2-fluoro-[1,1'-biphenyl]-4-yl)propanamide Structure](https://image.chemsrc.com/caspic/085/87657-78-1.png)