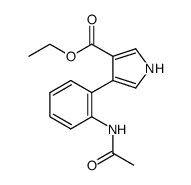
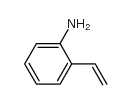
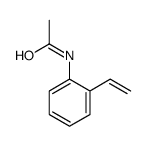
|
~45% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
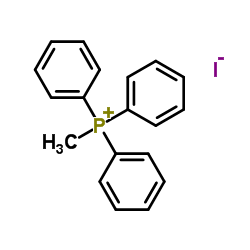
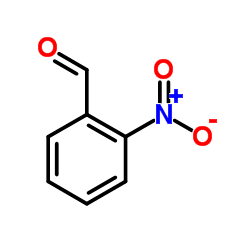
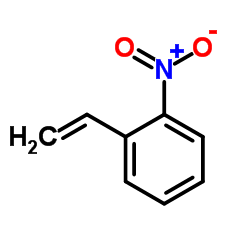

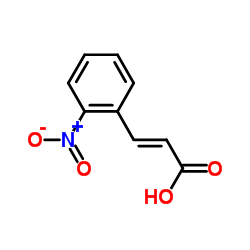
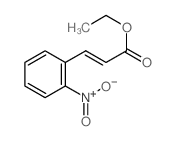

![4-methyl-3H-pyrrolo[2,3-c]quinoline Structure](https://image.chemsrc.com/caspic/393/920317-36-8.png)