|
~21% |
|
~71% |
|
~9% |
|
~93% |
|
~26% |
|
~% |
|
~% |
|
~86% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~44% |
|
~87% |
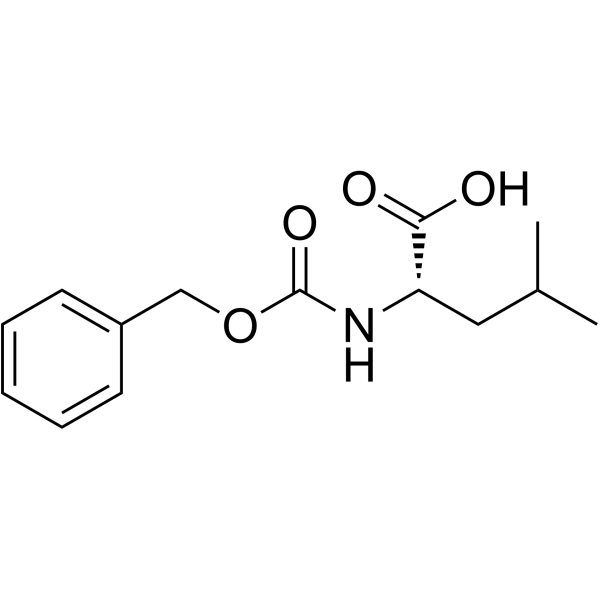
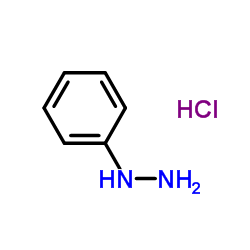
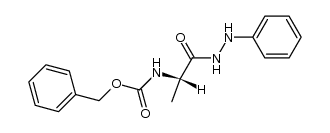
![L-Leucine,N-[(phenylmethoxy)carbonyl]-, 2-phenylhydrazide Structure](https://image.chemsrc.com/caspic/164/3242-70-4.png)
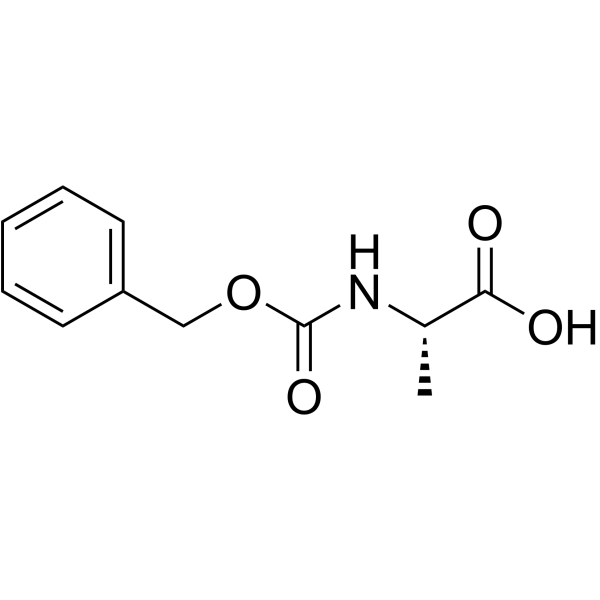

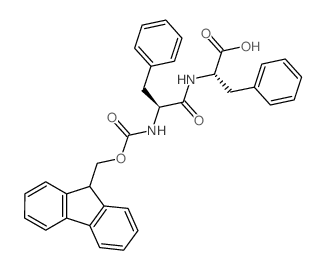
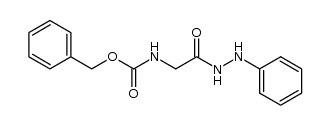

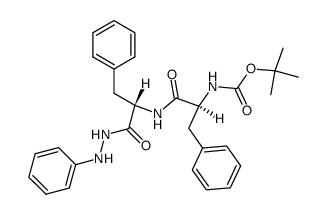
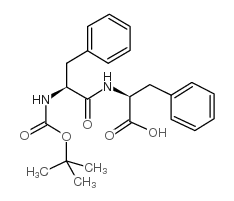

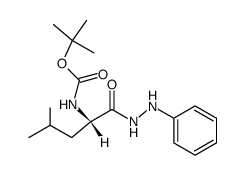
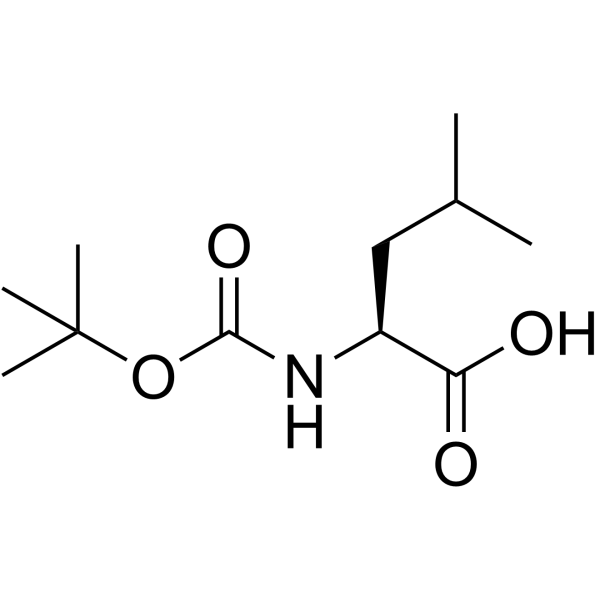
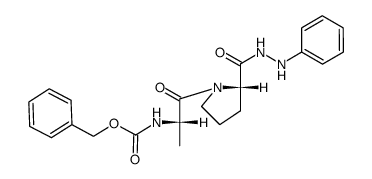
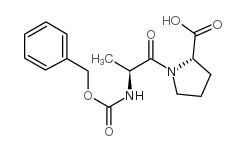

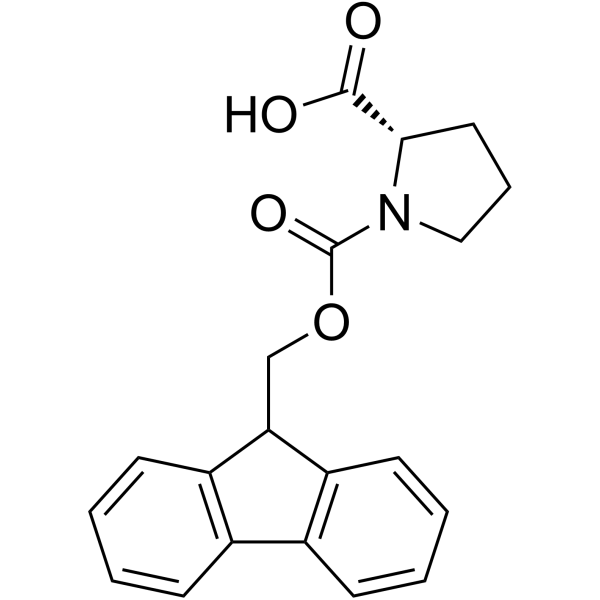
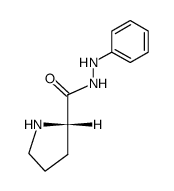
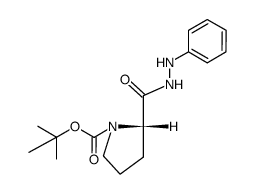
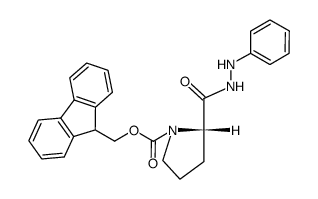
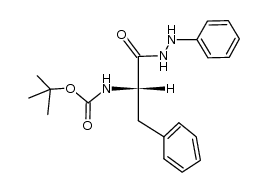

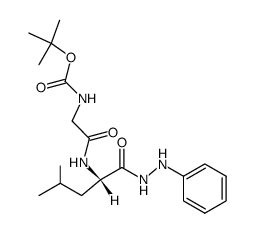
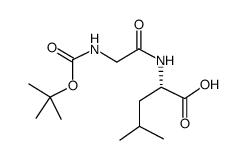
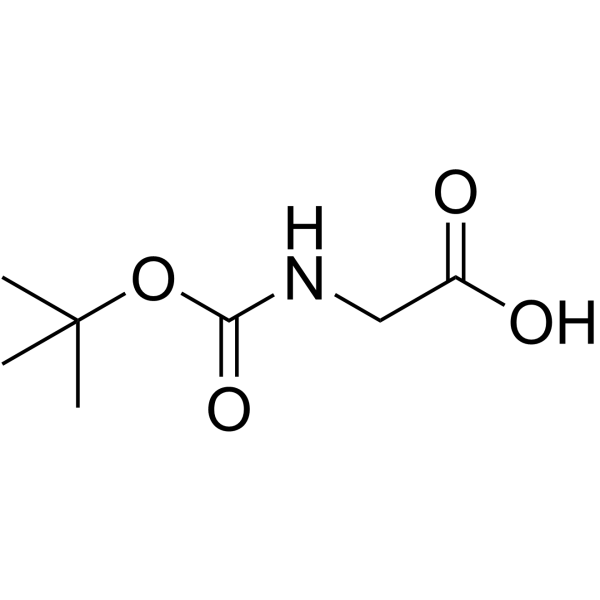
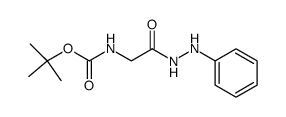
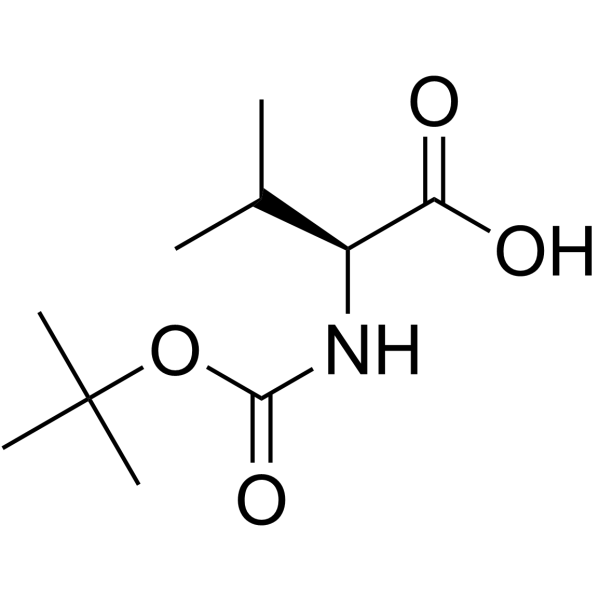
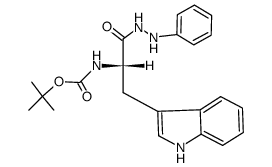
![N-[(tert-Butoxy)carbonyl]-L-tryptophan Structure](https://image.chemsrc.com/caspic/447/13139-14-5.png)