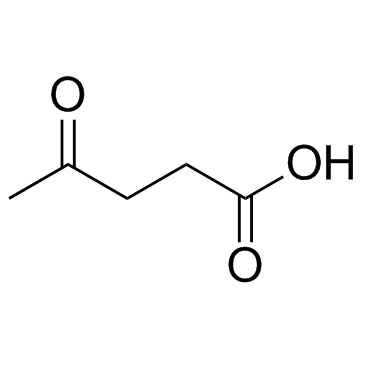
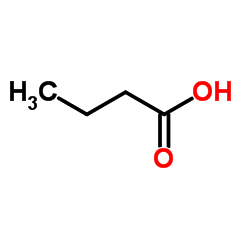
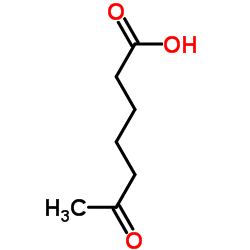
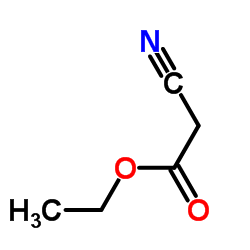
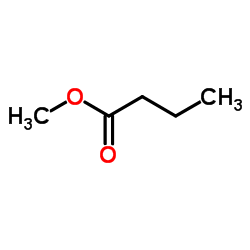
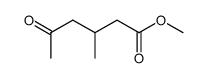
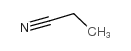
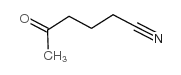
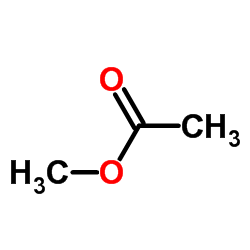
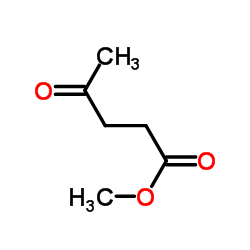
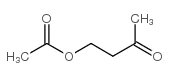
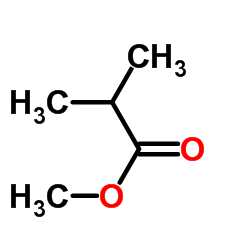
|
~10% |
|
~55% |
|
~59% |
|
~% |
|
~32% |
|
~% |
|
~52% |
|
~15% |
|
~14% |