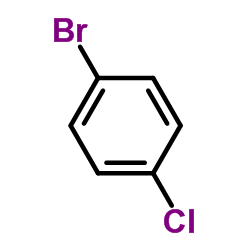
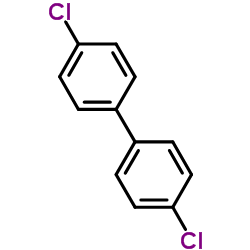
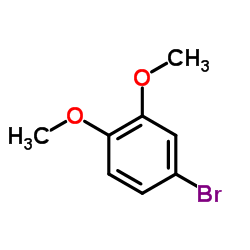
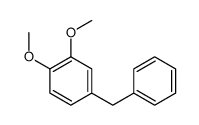
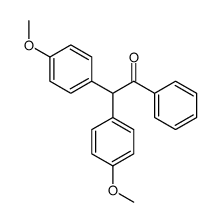
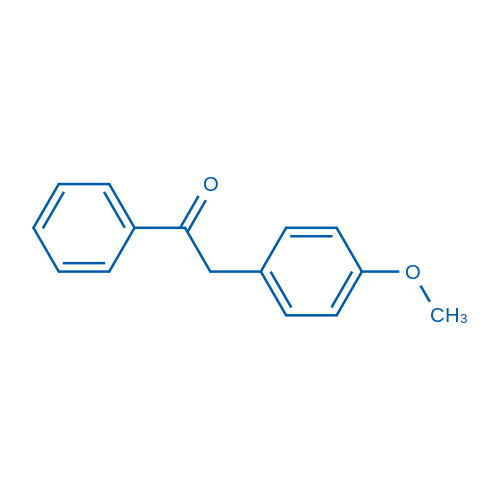
|
~90% |
|
~86% |
|
~96% |
|
~94% |
|
~83% |
|
~% |
|
~97% |
|
~48% |
|
~95% |
|
~88% |
|
~92% |
|
~% |
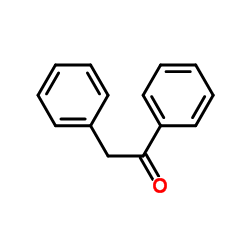
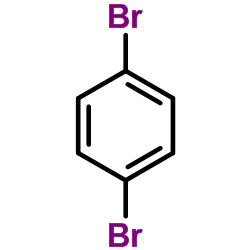
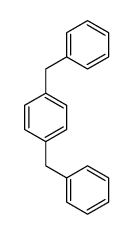
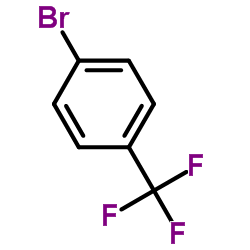
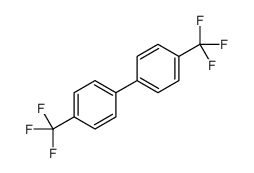
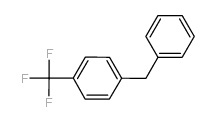
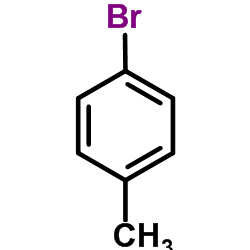
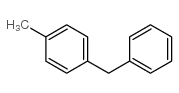
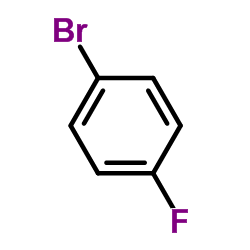
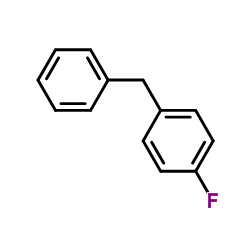
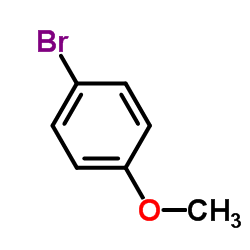
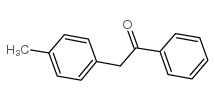
![1-methoxy-4-[(4-methylphenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/118/22865-60-7.png)


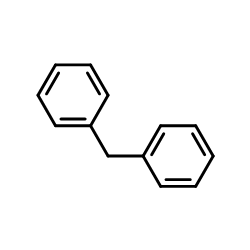
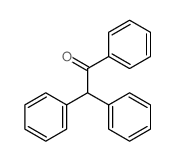
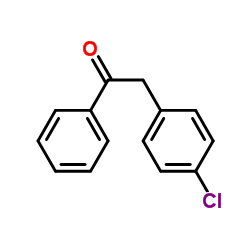
![BENZENE, 1-CHLORO-4-[(4-METHYLPHENYL)METHYL] Structure](https://image.chemsrc.com/caspic/334/30203-87-3.png)