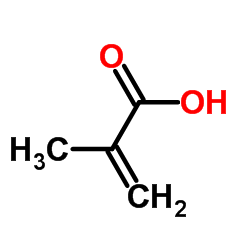
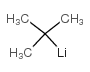
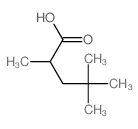
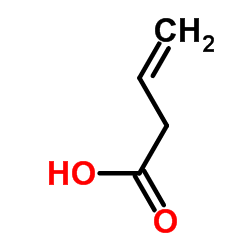
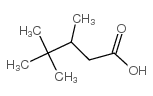
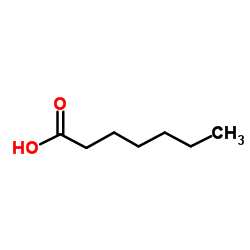
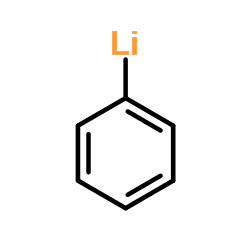
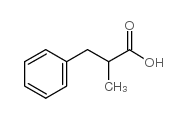
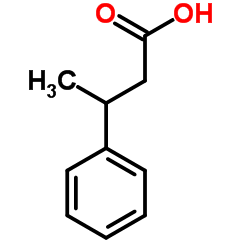
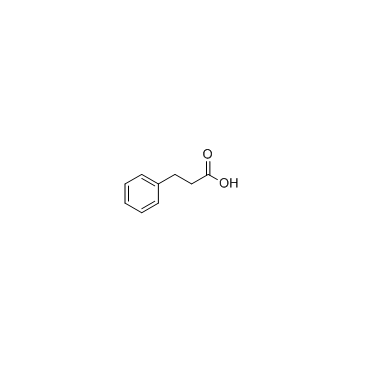
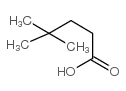
|
~73% |
|
~68% |
|
~43% |
|
~11% |
|
~77% |
|
~32% |
|
~57% |