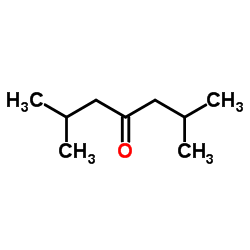
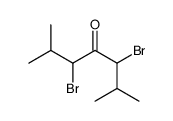
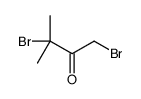
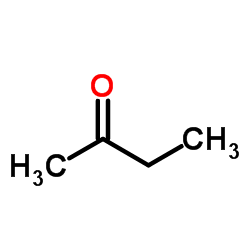
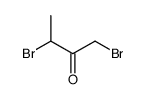
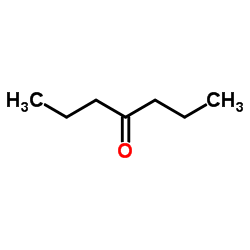
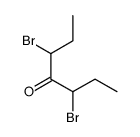
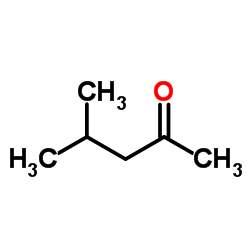
|
~71% |
|
~81% |
|
~75% |
|
~73% |
|
~83% |
|
~86% |