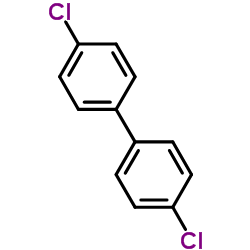
|
~61% |
|
~86% |
|
~70% |
|
~39% |
|
~58% |
|
~25% |
|
~52% |
|
~44% |
|
~79% |
|
~65% |
|
~18% |
|
~88% |
|
~13% |
|
~62% |

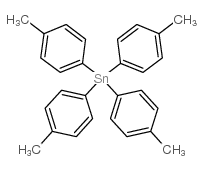

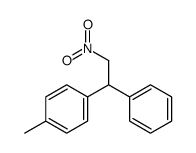
![1-methyl-4-[1-(4-methylphenyl)-2-nitroethyl]benzene Structure](https://image.chemsrc.com/caspic/236/85078-26-8.png)
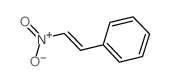

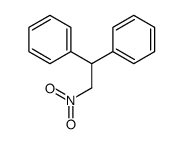
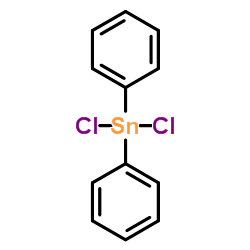
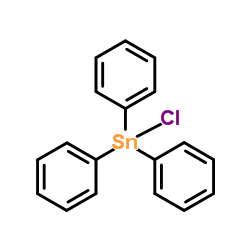
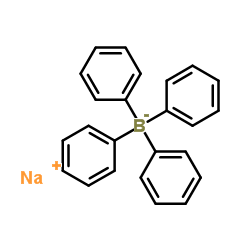
![2-[(E)-2-Nitrovinyl]thiophene Structure](https://image.chemsrc.com/caspic/350/34312-77-1.png)