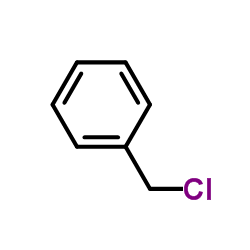
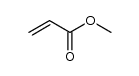
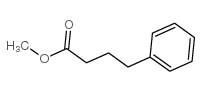
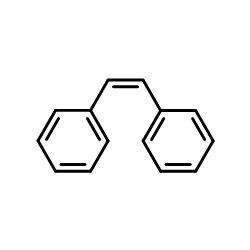
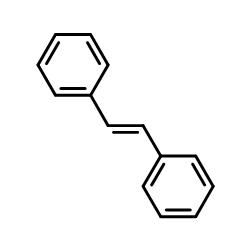
|
~73% |
|
~20% |
|
~77% |
|
~% |
|
~66% |
|
~0% |
|
~0% |
|
~69% |
|
~0% |
|
~68% |
|
~0% |
|
~77% |
|
~74% |
|
~74% |
|
~74% |
|
~16% |
|
~0% |

![1-(trifluoromethyl)-3-[2-[3-(trifluoromethyl)phenyl]ethyl]benzene Structure](https://image.chemsrc.com/caspic/057/72390-22-8.png)

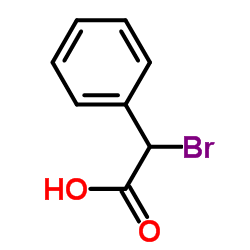
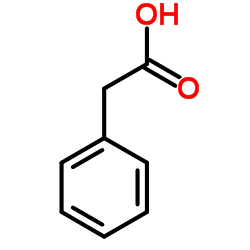
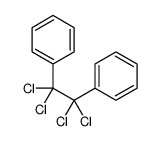
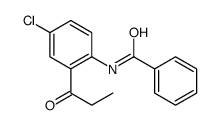

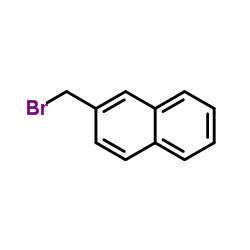
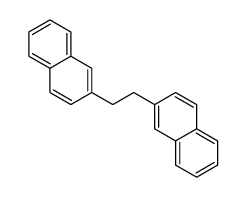
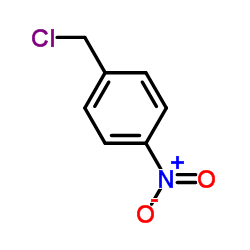
![1-nitro-4-[2-(4-nitrophenyl)ethyl]benzene Structure](https://image.chemsrc.com/caspic/400/736-30-1.png)

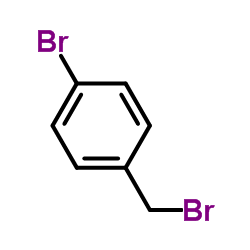
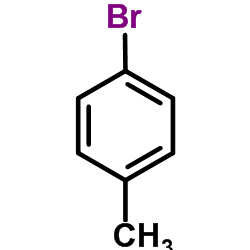
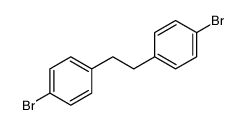
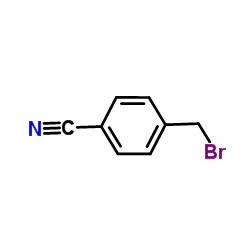
![4-[2-(4-cyanophenyl)ethyl]benzonitrile Structure](https://image.chemsrc.com/caspic/200/4381-02-6.png)

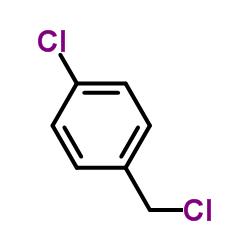
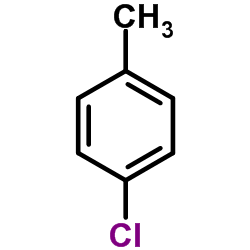
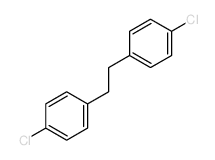

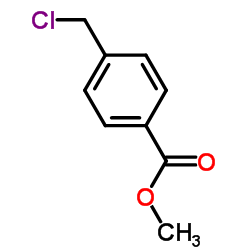
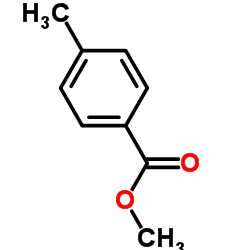
![methyl 4-[2-(4-methoxycarbonylphenyl)ethyl]benzoate Structure](https://image.chemsrc.com/caspic/017/797-21-7.png)