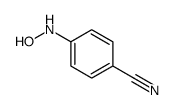
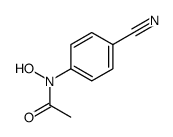
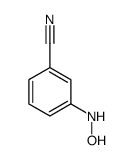
|
~% |
|
~% |
|
~% |
|
~% |
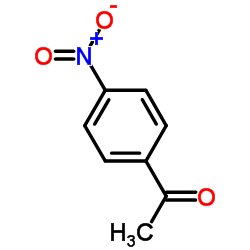
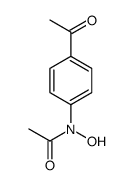
![Ethanone,1-[4-(hydroxyamino)phenyl] Structure](https://image.chemsrc.com/caspic/377/10517-47-2.png)