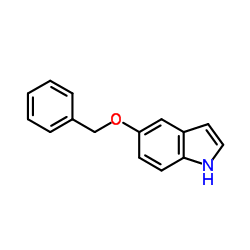
|
~92% |
|
~88% |
|
~77% |
|
~80% |
|
~31% |
|
~93% |
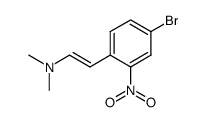
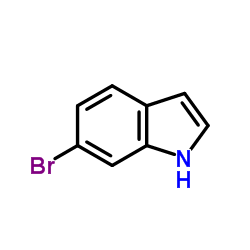
![1-[(E)-2-(5-methoxy-2-nitrophenyl)vinyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/427/61293-32-1.png)
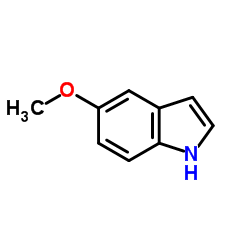
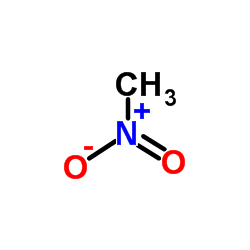
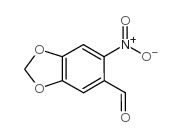

![1-[(E)-2-(4-methyl-2-nitrophenyl)vinyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/225/1335101-94-4.png)
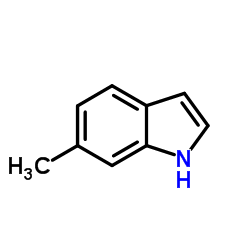


![1-[2-(5-Benzyloxy-2-nitrophenyl)vinyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/362/153805-85-7.png)