|
~56% |
|
~% |
|
~64% |
|
~% |
|
~% |
|
~% |
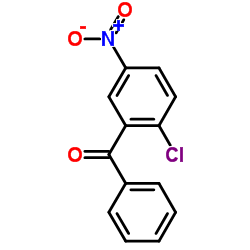
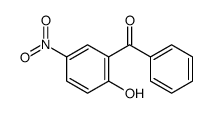
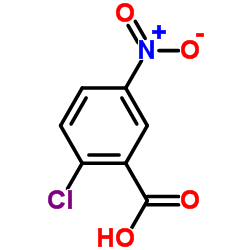
![6-[(hydroxyamino)-phenylmethylidene]-4-nitrocyclohexa-2,4-dien-1-one Structure](https://image.chemsrc.com/caspic/283/87974-54-7.png)
