|
~62% |
|
~89% |
|
~23% |
|
~8% |
|
~8% |
|
~63% |
|
~67% |
|
~86% |
|
~90% |
|
~33% |
|
~74% |
|
~91% |
|
~92% |
|
~35% |

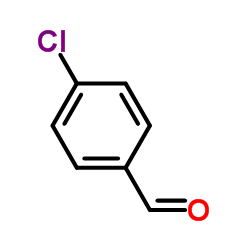
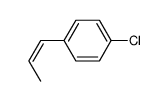
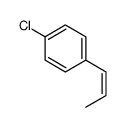


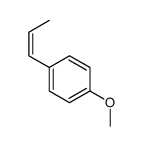
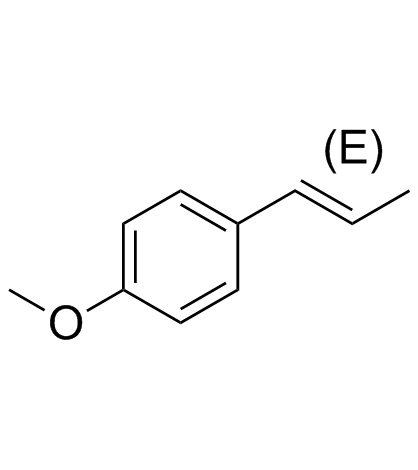
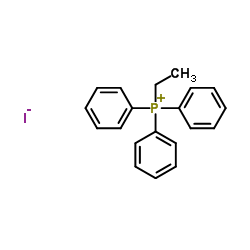
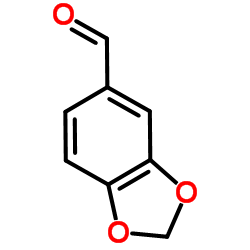

![5-[(E)-prop-1-enyl]benzo[1,3]dioxole Structure](https://image.chemsrc.com/caspic/439/4043-71-4.png)