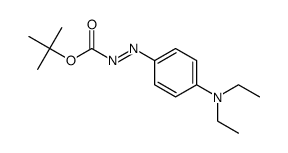
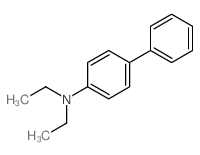
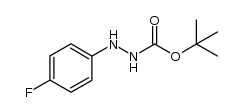
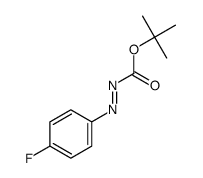
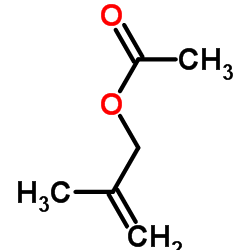
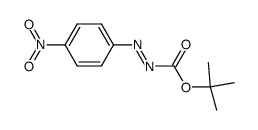
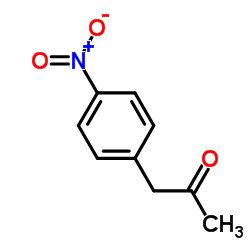
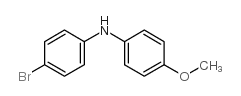
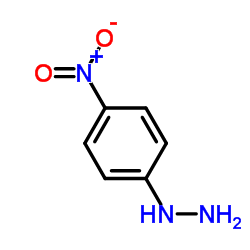
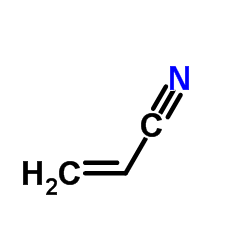
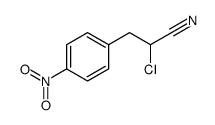
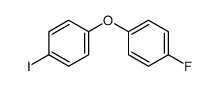
|
~49% |
|
~% |
|
~% |
|
~% |
|
~60% |
|
~42% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~59% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~65% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~77% |
|
~% |