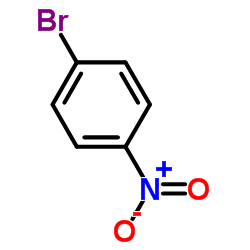
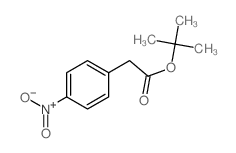
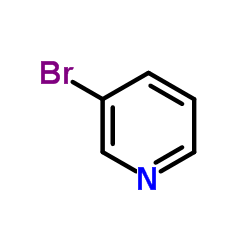
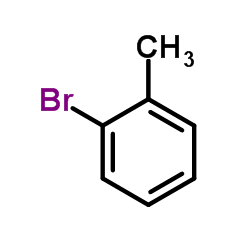
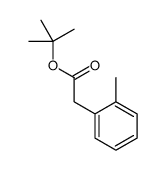
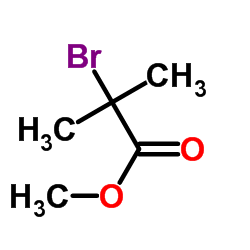
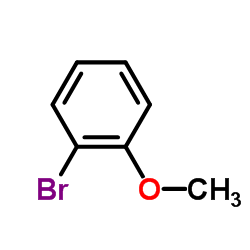
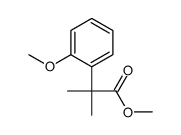
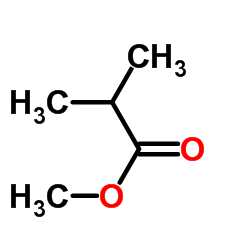
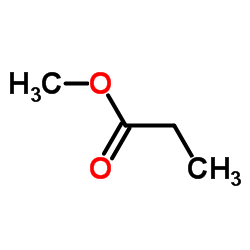
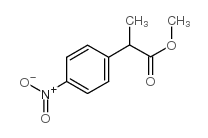
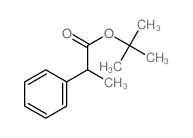
|
~92% |
|
~87% |
|
~88% |
|
~97% |
|
~92% |
|
~71% |
|
~99% |