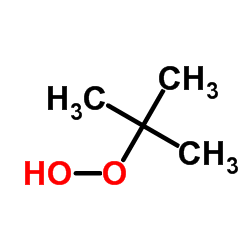
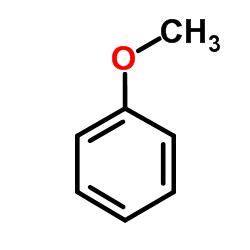
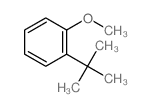
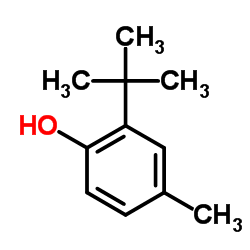
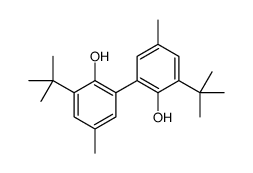
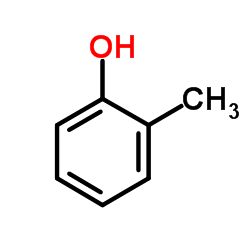
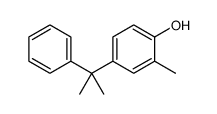
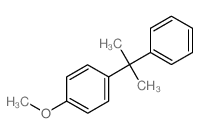
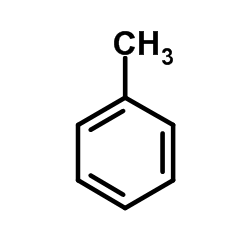
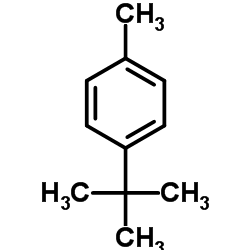
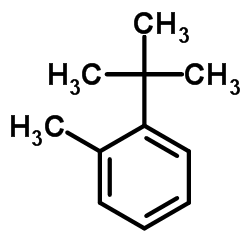
|
~11% |
|
~93% |
|
~% |
|
~49% |
|
~67% |
|
~64% |
|
~18% |