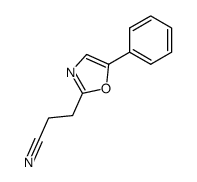
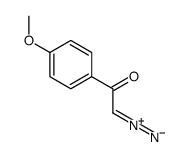
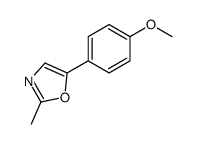
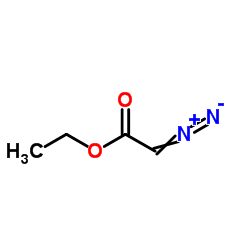
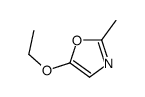
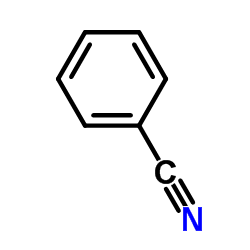
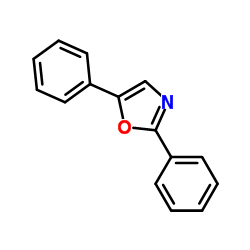
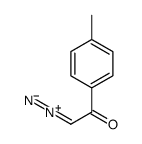
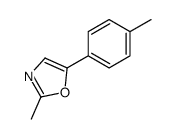
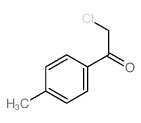
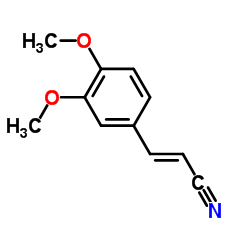
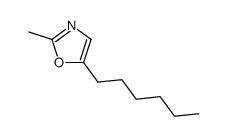
|
~98% |
|
~% |
|
~3% |
|
~91% |
|
~30% |
|
~73% |
|
~0% |
|
~% |
|
~94% |
|
~0% |



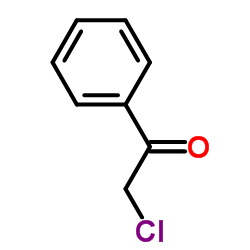
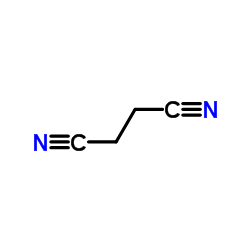
![5-phenyl-2-[2-(5-phenyl-1,3-oxazol-2-yl)ethyl]-1,3-oxazole Structure](https://image.chemsrc.com/caspic/011/31995-37-6.png)