柠檬烯环氧化物
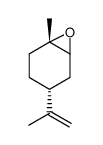
柠檬烯环氧化物结构式

|
常用名 | 柠檬烯环氧化物 | 英文名 | (+)-Trans-limonene1,2-epoxide |
|---|---|---|---|---|
| CAS号 | 6909-30-4 | 分子量 | 152.23300 | |
| 密度 | 0.930 g/mL at 20ºC(lit.) | 沸点 | N/A | |
| 分子式 | C10H16O | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | 65ºC |
| 中文名 | (+)-反式-柠檬烯 1,2-环氧化物 |
|---|---|
| 英文名 | (4R)-limonene 1α,2α-epoxide |
| 中文别名 | 柠檬烯环氧化物 |
| 英文别名 | 更多 |
| 密度 | 0.930 g/mL at 20ºC(lit.) |
|---|---|
| 分子式 | C10H16O |
| 分子量 | 152.23300 |
| 闪点 | 65ºC |
| 精确质量 | 152.12000 |
| PSA | 12.53000 |
| LogP | 2.52010 |
| InChIKey | CCEFMUBVSUDRLG-BBBLOLIVSA-N |
| SMILES | C=C(C)C1CCC2(C)OC2C1 |
| 外观性状 | 无色至浅黄色液体 |
| 折射率 | n20/D 1.466 |
| 储存条件 | 2-8°C |
| 个人防护装备 | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| 危害码 (欧洲) | F |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| 海关编码 | 2932999099 |
| 柠檬烯环氧化物上游产品 0 | |
|---|---|
| 柠檬烯环氧化物下游产品 1 | |
| 海关编码 | 2932999099 |
|---|---|
| 中文概述 | 2932999099. 其他仅含氧杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Catalytic mechanism of limonene epoxide hydrolase, a theoretical study.
J. Am. Chem. Soc. 127(41) , 14339-47, (2005) The catalytic mechanism of limonene epoxide hydrolase (LEH) was investigated theoretically using the density functional theory method B3LYP. LEH is part of a novel limonene degradation pathway found i... |
|
|
QM/MM study of the mechanism of enzymatic limonene 1,2-epoxide hydrolysis.
Biochim. Biophys. Acta 1824(2) , 263-8, (2012) Limonene 1,2-epoxide hydrolase (LEH) is completely different from those of classic epoxide hydrolases (EHs) which catalyze the hydrolysis of epoxides to vicinal diols. A novel concerted general acid c... |
|
|
Total synthesis of (+)-cymbodiacetal: a re-evaluation of the biomimetic route.
J. Org. Chem. 75(24) , 8465-70, (2010) A total synthesis of (+)-cymbodiacetal (1) has been completed from (R)-(+)-limonene oxide using a hetero-Diels-Alder cycloaddition as a key step. The key Diels-Alder cycloaddition proceeds with endo-s... |
| (1S,2R,4R)-4-Isopropenyl-1-methyl-1-cyclohexene 1,2-epoxide |
| D-LIMONENE 1,2-EPOXIDE |
| (1S,2R,4R)-limonene 1,2-oxide |
| (1S,4R)-1,2-Epoxy-p-menth-8-ene |
| (1R,4S)-trans-limonene oxide |
| (4R)-limonene 1alpha,2alpha-epoxide |
| (+)-trans-Limonene oxide |
| (1S,2R,4R)-limonene-1,2-epoxide |
| (+)-trans-p-Mentha-1,8-diene 1,2-epoxide |
| (+)-trans-Limonene 1,2-epoxide |
| (1S,2R,4R)-trans-1,2-limonene epoxide |
| (1S,2R)-1,2-epoxy-p-menth-8-ene |
| (1R,3R,6S)-6-methyl-3-prop-1-en-2-yl-7-oxabicyclo[4.1.0]heptane |
| 2a2g |
| trans-d-Limonene oxide |
| limonene oxide |
| LEO |
 CAS号22972-51-6
CAS号22972-51-6
