Ibiglustat succinate
更新时间:2025-08-27 13:20:07
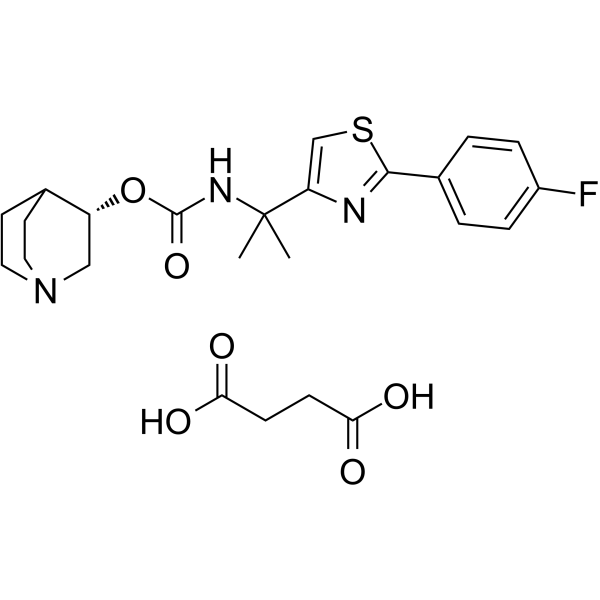
Ibiglustat succinate结构式

|
常用名 | Ibiglustat succinate | 英文名 | Ibiglustat succinate |
|---|---|---|---|---|
| CAS号 | 1629063-80-4 | 分子量 | 507.57 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C24H30FN3O6S | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
Ibiglustat succinate用途Ibiglustat(文格司他)琥珀酸盐是一种口服活性脑渗透葡糖神经酰胺合酶(GCS)抑制剂。琥珀酸依比鲁司他酯可用于研究高切病3型、与GBA突变相关的帕金森病、法布里病、GM2神经节苷脂病和常染色体显性多囊肾病[1][2]。 |
| 英文名 | Ibiglustat succinate |
|---|
| 描述 | Ibiglustat(文格司他)琥珀酸盐是一种口服活性脑渗透葡糖神经酰胺合酶(GCS)抑制剂。琥珀酸依比鲁司他酯可用于研究高切病3型、与GBA突变相关的帕金森病、法布里病、GM2神经节苷脂病和常染色体显性多囊肾病[1][2]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 依比格司他(SAR402671)琥珀酸盐(1μM,15天;法布里病(FD)细胞)在GL-3水平接近未经处理的WT细胞的生理水平,表明依比格司他琥珀酸盐可以防止额外的GL-3积累,并有助于改善FD心肌细胞中这种鞘脂的丰富水平[4]。 |
| 参考文献 |
| 分子式 | C24H30FN3O6S |
|---|---|
| 分子量 | 507.57 |
| InChIKey | TWRYSLPYKQOKAO-PKLMIRHRSA-N |
| SMILES | CC(C)(NC(=O)OC1CN2CCC1CC2)c1csc(-c2ccc(F)cc2)n1.O=C(O)CCC(=O)O |


