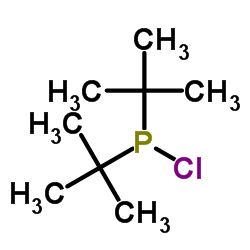
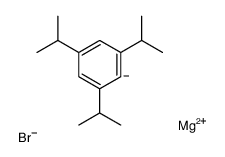
2-(二叔丁基膦)-3,6-二甲氧基-2'-4'-6'三-1-丙基-1,1'-双苯基
2-(二叔丁基膦)-3,6-二甲氧基-2'-4'-6'三-1-丙基-1,1'-双苯基结构式

|
常用名 | 2-(二叔丁基膦)-3,6-二甲氧基-2'-4'-6'三-1-丙基-1,1'-双苯基 | 英文名 | Di-tert-butyl(2',4',6'-triisopropyl-3,6-dimethoxybiphenyl-2-yl)phosphine |
|---|---|---|---|---|
| CAS号 | 1160861-53-9 | 分子量 | 484.693 | |
| 密度 | N/A | 沸点 | 534.9±50.0 °C at 760 mmHg | |
| 分子式 | C31H49O2P | 熔点 | 171 °C | |
| MSDS | 中文版 美版 | 闪点 | 347.6±30.4 °C |
用途有机膦配体 |
| 中文名 | 2-二叔丁基膦-2′,4′,6′-三异丙基-3,6-二甲氧基-1,1′-联苯 |
|---|---|
| 英文名 | ditert-butyl-[3,6-dimethoxy-2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane |
| 中文别名 | 2-(二叔丁基膦)-3,6-二甲氧基-2'-4'-6'三-1-丙基-1,1'-双苯基 |
| 英文别名 | 更多 |
| 沸点 | 534.9±50.0 °C at 760 mmHg |
|---|---|
| 熔点 | 171 °C |
| 分子式 | C31H49O2P |
| 分子量 | 484.693 |
| 闪点 | 347.6±30.4 °C |
| 精确质量 | 484.347015 |
| PSA | 32.05000 |
| LogP | 10.28 |
| 外观性状 | 固体;White to Light yellow powder to crystal |
| 蒸汽压 | 0.0±1.4 mmHg at 25°C |
| 储存条件 | 存放于惰性气体之中;避免空气 |
| 危害码 (欧洲) | Xi |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
|
~18% 
2-(二叔丁基膦)-3,6-二... 1160861-53-9 |
| 文献:MASSACHUSETTS INSTITUTE OF TECHNOLOGY Patent: WO2009/76622 A2, 2009 ; Location in patent: Page/Page column 116 ; |
|
~86% 
2-(二叔丁基膦)-3,6-二... 1160861-53-9 |
| 文献:Hoshiya, Naoyuki; Buchwald, Stephen L. Advanced Synthesis and Catalysis, 2012 , vol. 354, # 10 p. 2031 - 2037 |
|
~53% 
2-(二叔丁基膦)-3,6-二... 1160861-53-9 |
| 文献:Fors, Brett P.; Dooleweerdt, Karin; Zeng, Qingle; Buchwald, Stephen L. Tetrahedron, 2009 , vol. 65, # 33 p. 6576 - 6583 |
|
~% 
2-(二叔丁基膦)-3,6-二... 1160861-53-9 |
| 文献:Advanced Synthesis and Catalysis, , vol. 354, # 10 p. 2031 - 2037 |
|
~% 
2-(二叔丁基膦)-3,6-二... 1160861-53-9 |
| 文献:Advanced Synthesis and Catalysis, , vol. 354, # 10 p. 2031 - 2037 |
|
Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands.
Acc. Chem. Res. 41 , 1461, (2008) The cores of many types of polymers, ligands, natural products, and pharmaceuticals contain biaryl or substituted aromatic structures, and efficient methods of synthesizing these structures are crucia... |
|
|
Biaryl phosphane ligands in palladium-catalyzed amination.
Angew. Chem. Int. Ed. Engl. 47 , 6338, (2008) Palladium-catalyzed amination reactions of aryl halides have undergone rapid development in the last 12 years, largely driven by the implementation of new classes of ligands. Biaryl phosphanes have pr... |
|
|
Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User's Guide.
Chem. Sci. 2 , 27, (2011) Dialkylbiaryl phosphines are a valuable class of ligand for Pd-catalyzed amination reactions and have been applied in a range of contexts. This review attempts to aid the reader in the selection of th... |
| T-BUTYLBRETTPHOS |
| 2-(Di-tert-butylphosphino)-2′,4′,6′- triisopropyl-3,6-dimethoxy-1,1′-biphenyl |
| tert-Butyl BrettPhos |
| Bis(2-methyl-2-propanyl)(2',4',6'-triisopropyl-3,6-dimethoxy-2-biphenylyl)phosphine |
| t-BuBrett Phos |
| Phosphine, [3,6-dimethoxy-2',4',6'-tris(1-methylethyl)[1,1'-biphenyl]-2-yl]bis(1,1-dimethylethyl)- |
| tBuBrettPhos |
| t-BuBrett-Phos |
| Di-tert-butyl(2',4',6'-triisopropyl-3,6-dimethoxybiphenyl-2-yl)phosphine |
| t-BuBrettPhos |