1304-76-3
| 中文名 | 氧化铋 |
|---|---|
| 英文名 | Bismuth trioxide |
| 中文别名 |
氧化铋
三氧化二铋 |
| 英文别名 |
bismuthousoxide
Dibismuth trioxide Bismuth(III) Bismuth oxide Dibismuttrioxid Bismuth yellow bismuth sesquioxide Bismuth Trioxide MFCD00003462 Bismite Bi2-O3 Bismuth(III) oxide EINECS 215-134-7 |
| 密度 | 8.9 |
|---|---|
| 沸点 | 1890°C |
| 熔点 | 825 °C |
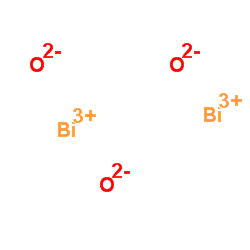
| 分子式 | Bi2O3 |
| 分子量 | 465.959 |
| 闪点 | 1890°C |
| 精确质量 | 465.945557 |
| 外观性状 | 黄色粉末 |
| 储存条件 | 保持贮藏器密封、储存在阴凉、干燥的地方,确保工作间有良好的通风或排气装置。 |
| 稳定性 | 1.如果遵照规格使用和储存则不会分解,未有已知危险反应,避免氧化物。 2.三氧化二铋纯品有α型和β型。α型为黄色单斜晶系结晶,相对密度8.9,熔点825 ℃,溶于酸,不溶于水和碱。β型为亮黄色至橙色,正方晶系,相对密度8.55,熔点860 ℃,溶于酸,不溶于水。容易被氢气、烃类等还原为金属铋。 |
| 水溶解性 | INSOLUBLE |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:256.965692 Da 8、标称质量:257 Da 9、平均质量:256.9791 Da |
| 计算化学 | 1、 氢键供体数量:0 2、 氢键受体数量:3 3、 可旋转化学键数量:0 4、 拓扑分子极性表面积(TPSA):3 5、 重原子数量:5 6、 表面电荷:0 7、 复杂度:0 8、 同位素原子数量:0 9、 确定原子立构中心数量:0 10、 不确定原子立构中心数量:0 11、 确定化学键立构中心数量:0 12、 不确定化学键立构中心数量:0 13、 共价键单元数量:5 |
| 更多 | 1. 性状:黄色重质粉末或单斜结晶。无气味。在空气中稳定。 2. 密度(g/mL,25/4℃):8.9 3. 熔点(ºC):825 4. 沸点(ºC,常压):1890 5. 闪点(ºC):18906. 溶解性:溶于盐酸和硝酸,不溶于水。加热变为褐红色,冷后仍变为黄色。 |
Synonym:Bismuth trioxide; Bismuthous oxide; Bismuth yellow Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Not available. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. Ingestion: May cause irritation of the digestive tract. May cause liver and kidney damage. Inhalation: May cause respiratory tract irritation. Chronic: May cause liver and kidney damage. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse. Ingestion: Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: Use extinguishing media most appropriate for the surrounding fire. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Storage: Store in a tightly closed container. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 1304-76-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to minimize contact with skin. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: yellow Odor: odorless pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 825 deg C Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Not available. Solubility in water: practically insoluble in water. Specific Gravity/Density: 8.8 Molecular Formula: Bi2O3 Molecular Weight: 465.96 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Dust generation. Incompatibilities with Other Materials: No significant incompatibilities identified with common materials and contaminants.. Hazardous Decomposition Products: None. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1304-76-3: EB2984460 LD50/LC50: CAS# 1304-76-3: Oral, mouse: LD50 = 10 gm/kg; Oral, rat: LD50 = 5000 mg/kg. Carcinogenicity: Bismuth trioxide - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: IMO Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: RID/ADR Shipping Name: Not regulated. Hazard Class: UN Number: Packing group: Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 1304-76-3: 1 Canada CAS# 1304-76-3 is listed on Canada's DSL List. CAS# 1304-76-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 1304-76-3 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 急性毒性:大鼠口经LD50:5gm/kg;小鼠口经LD50:10gm/kg 生态学数据: 通常来说对水是无害的,若无政府许可,勿将材料排入周围环境。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| 危害码 (欧洲) | Xi: Irritant; |
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | S26-S36/37 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 2 |
| RTECS号 | EB2984460 |
| 海关编码 | 8106009090 |
| 上游产品 8 | |
|---|---|
| 下游产品 10 | |
| 海关编码 | 8106009090 |
|---|











 灼烧结束,冷却后全部转变为柠檬黄色,即为成品。 6. 用 少 量 稀 硝 酸 洗16kg99.9%的工业铋表面,再用电导水洗去表面硝酸。然后加入1:1的高纯硝
灼烧结束,冷却后全部转变为柠檬黄色,即为成品。 6. 用 少 量 稀 硝 酸 洗16kg99.9%的工业铋表面,再用电导水洗去表面硝酸。然后加入1:1的高纯硝