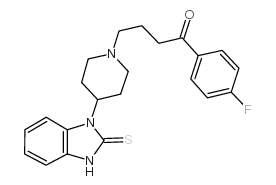
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
EL7570000
-
CHEMICAL NAME :
-
1-Butanone, 4-(4-(2,3-dihydro-2-thioxo-1H-benzimidazol-1-yl)-1-pi peridinyl)-1- (4-fluorophenyl)-
-
CAS REGISTRY NUMBER :
-
57648-21-2
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
14
-
MOLECULAR FORMULA :
-
C22-H24-F-N3-O-S
-
MOLECULAR WEIGHT :
-
397.55
-
WISWESSER LINE NOTATION :
-
T56 BMYNJ CUS D- DT6NTJ A3VR DF
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
232 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 27(Suppl),124P,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,647,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>12100 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,647,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 21,1116,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
85 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,647,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
20 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,647,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
237 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,647,1990 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
36 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Blood - changes in bone marrow (not otherwise specified) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13(Suppl 5),S1177,1985 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
33 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 12,861,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
1600 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 32,34B,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
17600 ug/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13(Suppl 5),S1265,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
13 mg/kg
-
SEX/DURATION :
-
female 17 day(s) after conception - 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13(Suppl 5),S1291,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
12800 ug/kg
-
SEX/DURATION :
-
female 0-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Embryo or Fetus - other effects to embryo Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13(Suppl 5),S1247,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5200 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 12,872,1981
|


![N-[1-[3-[2-(4-fluorophenyl)-1,3-dioxolan-2-yl]propyl]piperidin-4-yl]benzene-1,4-diamine结构式](https://image.chemsrc.com/caspic/290/57718-48-6.png)
![4-[(2-Aminophenyl)amino]-1-[3-[2-(4-fluorophenyl)-1,3-dioxolan-2-yl]propyl]pyridinium Chloride结构式](https://image.chemsrc.com/caspic/084/65053-27-2.png)