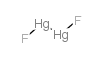
13967-25-4
| 中文名 | 氟化亚汞(I) |
|---|---|
| 英文名 | mercury(i) fluoride |
| 中文别名 | 氟化汞 |
| 英文别名 |
MERCUROUS FLUORIDE
EINECS 237-747-9 Mercuryfluoride1 MFCD00016136 dimercury difluoride |
| 密度 | 8.73 g/mL at 25 °C(lit.) |
|---|---|
| 沸点 | 19.5ºC at 760mmHg |
| 熔点 | 570°C |
| 分子式 | F2Hg2 |
| 分子量 | 439.17700 |
| 精确质量 | 441.93800 |
| LogP | 0.83540 |
| 储存条件 | 常温常压下稳定 避免的物料:水分/潮湿 氧化物 酸 |
| 稳定性 | 常温常压下稳定 避免的物料:水分/潮湿 氧化物 酸 在约240℃时升华。具有感光性,遇光或接触氨气即变黑。极微量地溶于水中,但用水能缓慢地将其分解为氧化汞和氟化氢。 |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:441.938006 Da 8、标称质量:442 Da 9、平均质量:439.1768 Da |
| 计算化学 | 1、 氢键供体数量:0 2、 氢键受体数量:2 3、 可旋转化学键数量:0 4、 拓扑分子极性表面积(TPSA):0 5、 重原子数量:3 6、 表面电荷:0 7、 复杂度:0 8、 同位素原子数量:0 9、 确定原子立构中心数量:0 10、 不确定原子立构中心数量:0 11、 确定化学键立构中心数量:0 12、 不确定化学键立构中心数量:0 13、 共价键单元数量:3 |
| 更多 | 1. 性状:黄色正方晶系的结晶。 2. 密度(g/mL,25/4℃):8.73 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):570 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):不适用的 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:分解 |
|
Section 1: Product Identification Chemical Name:Mercury (I) fluoride, 95+% CAS Registry Number:13967-25-4 Formula:Hg2F2 EINECS Number:237-747-9 Chemical Family:metal halide Synonym:Mercurous fluoride
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound13967-25-4100%.025mg/m3 (as Hg)0.05mg/m3 (as Hg) Section 3: Hazards Identification Mercury salts are poisonous by ingestion and inhalation of dust. Acute effects include kidney failure. Emergency Overview: Prolonged exposure may cause heritable genetic damage and impaired fertility. Primary Routes of Exposure:Ingestion, skin, Inhalation of dust. Eye Contact:May cause mild to severe irritation of the eyes. May deposit mercury in the lens of the eye. Skin Contact:May cause mild to severe irritation of the skin. Toxic in contact with skin. Toxic by inhalation. Dust may cause severe respiratory and gastrointestinal distress, and may damage the Inhalation: central nervous system. Very toxic if swallowed. Lack of appetite, abdominal cramps, vomiting, kidney failure, paralysis. Mercury binds Ingestion: to thiol groups of protein. Poison. Stomach upset and pain, mouth pain, vomiting, excessive salivation, urine production stops, urine Acute Health Affects: products appear in the blood, ataxia (difficulty in moving). The chronic effects of mercury poisoning include stomatitis, tremors, psychic disturbances, excessive Chronic Health Affects: salivation, dermatitis, and pain on chewing. May cause heritable genetic damage and impair fertility. NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with water. Remove contaminated clothes if necessary. Apply calcium gluconate jelly Skin Exposure: or water soluble calcium salts as antidote. Seek medical assistance. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce Ingestion: vomiting only if directed by medical personnel. SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:none Explosion Limits:none Extinguishing Medium:None. Material is non-flammable. If this product is involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure Special Fire Fighting Procedures: self-contained breathing apparatus and full protective clothing. Hazardous Combustion andIf involved in a fire this material may emit corrosive fumes of hydrofluoric acid and toxic mercury salts. Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures Small spills can be mixed with powdered sodium bicarbonate, lime, or calcium carbonate and swept up. Avoid Spill and Leak Procedures:raising dust. Spillage in areas not adequately ventilated may require an evacuation of area. Emergency response teams will require self-contained breathing apparatus. SECTION 7: Handling and Storage Handling and Storage:Store in a tightly sealed non-glass container away from moisture. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear protective clothing and gloves. Consult with glove manufacturer to determine the proper type of glove. Ventilation:solid may form a toxic fine dust. If possible, handle the solid in an efficient fume hood. If in form of fine dust and ventilation is not available a respirator should be worn. The use of respirators Respirator: requires a Respirator Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:solid may form a toxic fine dust. If possible, handle the solid in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:white pwdr. Molecular Weight:439.22 Melting Point:570° Boiling Point:no data Vapor Pressure:no data Specific Gravity:8.73 Odor:none Solubility in Water:insoluble SECTION 10: Stability and Reactivity Stability:air and moisture stable Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:none Incompatibility:active metals and strong mineral acids Decomposition Products:none SECTION 11: Toxicological Information RTECS Data:No information available in the RTECS files. Carcinogenic Effects:no data Mutagenic Effects:no data Tetratogenic Effects:no data SECTION 12: Ecological Information Avoid release to groundwater or waterways. Very toxic to aquatic organisms. May cause long-term adverse Ecological Information: effects. SECTION 13: Disposal Considerations Disposal:Dispose of according to local, state and federal regulations. SECTION 14: Transportation Shipping Name (CFR):Mercury compounds, solid, N.O.S. Hazard Class (CFR):6.1 Additional Hazard Class (CFR):NA Packaging Group (CFR):II UN ID Number (CFR):UN# 2025 Shipping Name (IATA):Mercury compound, solid, N.O.S. Hazard Class (IATA):6.1 Additional Hazard Class (IATA):NA Packaging Group (IATA):II UN ID Number (IATA):UN# 2025 SECTION 15: Regulatory Information TSCA:Not listed in the TSCA inventory. SARA (Title 313):Title compound: See Category Code N458 for reporting. Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 1、急性毒性:主要的刺激性影响: 在皮肤上面:刺激皮肤和粘膜; 在眼睛上面:刺激的影响;没有已知的敏化影响。 生态学数据: 对水体是极其危害的,即使小量产品不能接触地下水,水道或污水系统,未经政府许可勿将材料排入周围环境。
|
| 危害码 (欧洲) | T+: Very toxic;N: Dangerous for the environment; |
|---|---|
| 风险声明 (欧洲) | R26/27/28 |
| 安全声明 (欧洲) | S13-S28-S45-S60-S61 |
| 危险品运输编码 | UN 2025 6.1/PG 2 |
| WGK德国 | 3 |
| 包装等级 | II |
| 危险类别 | 6.1 |
| 上游产品 0 | |
|---|---|
| 下游产品 1 | |