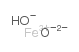20344-49-4
| 中文名 | 水合氧化铁(III) |
|---|---|
| 英文名 | Iron hydroxide oxide |
| 中文别名 |
氧化铁(III)
γ-氢氧化铁(III) 针铁矿 |
| 英文别名 |
Iron(III) hydroxide
dodecacarbonyl-triangulo-tri-iron ferric acid ferric oxide yellow iron dodecarbonyl dodecacarbonyltriiron(0) tri-iron dodecacarbonyl iron oxide yellow EINECS 243-746-4 dodecacarbonyltriiron MFCD00016080 iron(III) oxide Ferric hydroxide oxide Ferric hydroxide oxide (Iron(III) oxide |
| 密度 | 3.4-3.9 |
|---|---|
| 熔点 | 135°C |
| 分子式 | FeHO2 |
| 分子量 | 88.85170 |
| 精确质量 | 88.93260 |
| PSA | 23.06000 |
| 外观性状 | 细,黄色,无气味的粉末 |
| 储存条件 | 放入紧密的贮藏器内,储存在阴凉,干燥的地方 |
| 稳定性 | 避免接触酸 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:1 3.氢键受体数量:2 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积37.3 7.重原子数量:3 8.表面电荷:0 9.复杂度:10.3 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:无气味棕色粉末 2.密度(g/mL,25/4℃):4.03 3.相对蒸汽密度(g/mL,空气=1):未确定 4.熔点(ºC):未确定 5.沸点(ºC,常压):未确定 6.沸点(ºC,5.2kPa):未确定 7.折射率:2.3 8.闪点(ºC):未确定 9.比旋光度(º):未确定 10.自燃点或引燃温度(ºC):未确定 11.蒸气压(kPa,25ºC):未确定 12.饱和蒸气压(kPa,60ºC):未确定 13.燃烧热(KJ/mol):未确定 14.临界温度(ºC):未确定 15.临界压力(KPa):未确定 16.油水(辛醇/水)分配系数的对数值:未确定 17.爆炸上限(%,V/V):未确定 18.爆炸下限(%,V/V):未确定 19.溶解性:未确定 |
|
Section1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE Product identifiers Product name: Iron(III) oxide CAS-No.: 20344-49-4 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances Section2. HAZARDS IDENTIFICATION Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC. Label elements The product does not need to be labelled in accordance with EC directives or respective national laws. Other hazards - none Section3. COMPOSITION/INFORMATION ON INGREDIENTS Substances Synonyms: Ferric hydroxide oxide Formula: HFeO2 Molecular Weight: 88,85 g/mol Section4. FIRST AID MEASURES Description of first aid measures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. In case of skin contact Wash off with soap and plenty of water. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Most important symptoms and effects, both acute and delayed Indication of any immediate medical attention and special treatment needed no data available Section5. FIREFIGHTING MEASURES Extinguishing media Suitable extinguishing media Use extinguishing measures that are appropriate to local circumstances and the surrounding environment. Special hazards arising from the substance or mixture Iron oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information The product itself does not burn. Section6. ACCIDENTAL RELEASE MEASURES Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapors, mist or gas. Environmental precautions No special environmental precautions required. Methods and materials for containment and cleaning up Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. Section7. HANDLING AND STORAGE Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Specific end uses no data available Section8. EXPOSURE CONTROLS/PERSONAL PROTECTION Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls General industrial hygiene practice. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Immersion protection Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: > 480 min Material tested:Dermatril® ( Z677272, Size M) Splash protection Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: > 30 min Material tested:Dermatril® ( Z677272, Size M) data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 873000, test method: EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an Industrial Hygienist familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Section9. PHYSICAL AND CHEMICAL PROPERTIES Information on basic physical and chemical properties a) AppearanceForm: crystalline Colour: red brown b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointnot applicable h) Evaporation rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Autoignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available Section10. STABILITY AND REACTIVITY Reactivity no data available Chemical stability no data available Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available Section11. TOXICOLOGICAL INFORMATION Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitization no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Potential health effects InhalationMay be harmful if inhaled. May cause respiratory tract irritation. IngestionMay be harmful if swallowed. SkinMay be harmful if absorbed through skin. May cause skin irritation. Eyes May cause eye irritation. Additional Information RTECS: Not available Section12. ECOLOGICAL INFORMATION Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment no data available Other adverse effects no data available Section13. DISPOSAL CONSIDERATIONS Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contaminated packaging Dispose of as unused product. Section14. TRANSPORT INFORMATION UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xn |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | - |