Phase I trial of ilmofosine as a 24 hour infusion weekly.
Patrick A Singleton, Nurbek Mambetsariev, Frances E Lennon, Biji Mathew, Jessica H Siegler, Liliana Moreno-Vinasco, Ravi Salgia, Jonathan Moss, Joe Gn Garcia
文献索引:Invest. New Drugs 13(3) , 205-10, (1995)
全文:HTML全文
摘要
Ilmofosine, an ether lipid derivative of lysophosphatidylcholine has antineoplastic activity in vitro and in vivo. Maximum efficacy in preclinical models is associated with prolonged exposure to the drug. In a Phase I trial of a weekly 2 hour infusion schedule of ilmofosine, a syndrome of lethargy, diminished performance status, and mild hepatotoxicity was dose-limiting at 550 mg/m2. To avoid the higher drug concentrations associated with a brief infusion, a Phase I study of a weekly 24 hour infusional schedule was undertaken in an attempt to maximize dose-intensity. Doses were escalated from 550 to 800 mg/m2. Toxicities included nausea, anorexia, fatigue, and minor elevations of liver function tests. The dose limiting toxicity at 800 mg/m2 was a syndrome of severe abdominal pain. No neutropenia or thrombocytopenia was observed except in one patient who was found to have a myelodysplastic syndrome, thought not to be related to drug therapy. The more prolonged infusion schedule of ilmofosine did not result in a substantial increase in the tolerable dose.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
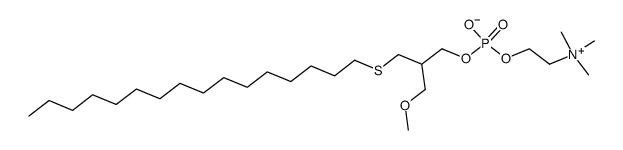 |
ILMOFOSINE
CAS:83519-04-4 |
C26H56NO5PS |
|
A phase II trial of ilmofosine in non-small cell bronchogeni...
1996-01-01 [Invest. New Drugs 14(2) , 219-22, (1996)] |
|
Structure-function relationships of alkyl-lysophospholipid a...
1993-06-01 [Lipids 28(6) , 511-6, (1993)] |
|
Quaternary ammonium analogs of ether lipids inhibit the acti...
1998-01-01 [Cancer Chemother. Pharmacol. 42(4) , 319-26, (1998)] |
|
Expression of two 50 kDa proteins is associated with sensiti...
1997-10-01 [Eur. J. Cancer 33(11) , 1875-80, (1997)] |
|
Cytotoxicity of ether phospholipid BM 41.440 on neuroblastom...
1995-01-01 [J. Cancer Res. Clin. Oncol. 121(5) , 262-6, (1995)] |