Palladium-catalyzed carbonylative annulation of o-alkynylphenols: syntheses of 2-substituted-3-aroyl-benzo[b]furans.
Youhong Hu, Yan Zhang, Zhen Yang, Reza Fathi
文献索引:J. Org. Chem. 67 , 2365, (2002)
全文:HTML全文
摘要
We report here a general synthetic methodology for palladium-catalyzed carbonylative annulation of o-alkynylphenol to construct 2-substituted-3-aroyl-benzo[b]furan. On the basis of the results, this methodology could be applied to a wider selection of iodide substrates to generate desired products. In accordance with mechanistic studies, this process involves coordination of cationic and less hindered acyl palladium complexes with o-alkynylphenols to create a desired cascade triad (coordination, nucleophilic addition, and reductive elimination). Consistent with this mechanism, addition of 1 equiv of AgBF(4) to palladium catalyst Pd(Ph(3)P)(4) generates an ideal candidate for this unique transformation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
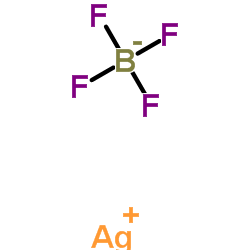 |
四氟硼酸银
CAS:14104-20-2 |
AgBF4 |
|
Development of a gold-multifaceted catalysis approach to the...
2013-02-01 [J. Org. Chem. 78(3) , 920-34, (2013)] |
|
Investigating silver coordination to mixed chalcogen ligands...
2012-01-01 [Molecules 17(11) , 13307-29, (2012)] |
|
[J. Mol. Catal. 79 , 85, (1993)] |
|
[Synlett , 1901, (2007)] |
|
Kende, A. S.; Huang, H.
[Tetrahedron Lett. 38 , 3353, (1997)] |