| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
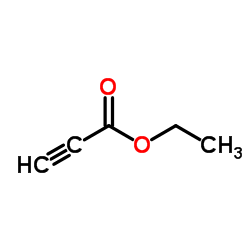 |
丙炔酸乙酯
CAS:623-47-2 |
|
 |
丁炔二酸二甲酯
CAS:762-42-5 |
|
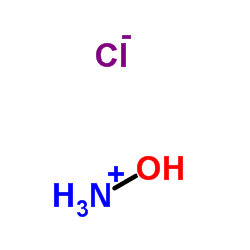 |
盐酸羟胺
CAS:5470-11-1 |
|
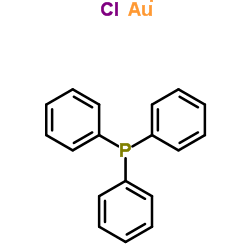 |
三苯基膦氯金
CAS:14243-64-2 |
|
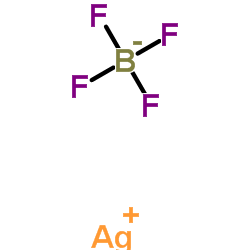 |
四氟硼酸银
CAS:14104-20-2 |