Effect of polyanion on the acidic conformational transition of native and denatured ferricytochrome c. Circular dichroism study.
E Sedlák, M Antalík
文献索引:Gen. Physiol. Biophys. 21(2) , 175-88, (2002)
全文:HTML全文
摘要
Interaction of polyanion poly(vinylsulfate) with oxidized cytochrome c (cyt c) significantly affects the protein main characteristics. One of them, pKa value of acidic transition, was shifted from an apparent pKa value 2.5 (typical for cyt c in low ionic strength solvent) to approximately 5.20 +/- 0.15 upon polyanion binding to the protein, pointing to a likely involvement of histidines 26 and/or 33 in the protein acidic transition in complex with the polyanion. The acidic transition followed at 6 different wavelengths all over circular dichroism spectrum, monitoring different parts of the protein structure, revealed basically two-state character process. Only ellipticity at 262 nm indicated a low-cooperative pH-induced conformational transition in heme region with an apparent pKa approximately 4.34 +/- 0.25 in accordance with absorbance change at 620 nm. Polyanion also interacts with chemically-denatured (in the presence of 9 mol/l urea) state of the protein as it follows from stabilization of protein residual structure at acidic pH and its effect on pKa value of acidic transition of chemically-denatured cyt c. Destabilization effect of polyanions on native and, on the other hand, stabilization influence on partially unfolded conformations of the protein are discussed with an implication for their chaperone-like properties in vivo and in vitro.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
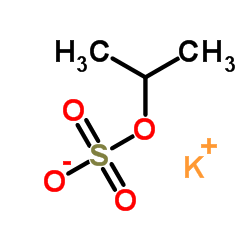 |
聚乙烯硫酸钾盐
CAS:26182-60-5 |
C3H7KO4S |
|
Binding of ethidium to the nucleosome core particle. 2. Inte...
1991-06-11 [Biochemistry 30 , 5644, (1991)] |
|
Apparent specificity of bovine seminal ribonucleases can dep...
1992-02-01 [Biochem. Int. 26(1) , 125-33, (1992)] |
|
A mycelium with polyelectrolyte complex-bunched hyphae: prep...
2007-04-15 [Colloids Surf. B Biointerfaces 56(1-2) , 155-60, (2007)] |
|
Coulombic and noncoulombic effect of polyanions on cytochrom...
1998-09-01 [Biopolymers 46(3) , 145-54, (1998)] |
|
Light scattering study of complex formation between protein ...
2007-04-15 [Colloids Surf. B Biointerfaces 56(1-2) , 142-8, (2007)] |