Light scattering study of complex formation between protein and polyelectrolyte at various ionic strengths.
Hiroshi Matsunami, Rie Kikuchi, Kazuyoshi Ogawa, Etsuo Kokufuta
文献索引:Colloids Surf. B Biointerfaces 56(1-2) , 142-8, (2007)
全文:HTML全文
摘要
Formation of protein-polyelectrolyte complexes (PPCs) between bovine serum albumin (BSA) and potassium poly (vinyl alcohol) sulfate (KPVS) was studied at pH 3 as a function of ionic strength. Turbidimetric titration was employed by a combination of dynamic light scattering (DLS) and electrophoretic light scattering (ELS). The formal charge (Z(PPC)) of the resulting PPCs at different ionic strengths were estimated from ELS data by assuming the free draining and the non-free draining model. The radius of a BSA molecule in the complex was used in the former model for calculation of Z(PPC) with the Henry's equation, while in the latter case the hydrodynamic radius of a PPC particle determined from DLS was employed. The results obtained were compared with the Z(PPC) values calculated using a relation of Z(PPC)=n(b)Z(BSA)+alphaZ(KPVS), where Z(BSA) (> or =0) and Z(KPVS) (< or =0) denote the formal charge of BSA and KPVS, respectively. Moreover, n(b) is the number of bound proteins per complex composed of alpha polymer chains. It was suggested that the PPC between BSA and KPVS behaves as a free draining molecule during the electrophoresis, at least at a high ionic strength. Also suggested is that the PPC formation at low ionic strength follows a 1:1 stoichiometry in the charge neutralization.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
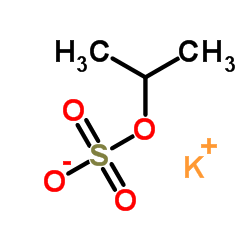 |
聚乙烯硫酸钾盐
CAS:26182-60-5 |
C3H7KO4S |
|
Binding of ethidium to the nucleosome core particle. 2. Inte...
1991-06-11 [Biochemistry 30 , 5644, (1991)] |
|
Apparent specificity of bovine seminal ribonucleases can dep...
1992-02-01 [Biochem. Int. 26(1) , 125-33, (1992)] |
|
A mycelium with polyelectrolyte complex-bunched hyphae: prep...
2007-04-15 [Colloids Surf. B Biointerfaces 56(1-2) , 155-60, (2007)] |
|
Coulombic and noncoulombic effect of polyanions on cytochrom...
1998-09-01 [Biopolymers 46(3) , 145-54, (1998)] |
|
Dichroic ATR-FTIR spectroscopy on oriented α-helical poly(l-...
2010-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 77(4) , 709-16, (2010)] |