Journal of Peptide Science
2001-03-01
Properties and applications of the (2-nitrofluoren-9-yl)methoxycarbonyl group.
B Henkel, E Bayer
文献索引:J. Pept. Sci. 7(3) , 152-6, (2001)
全文:HTML全文
摘要
This paper presents a new protecting group, the (2-nitrofluoren-9-yl)methoxycarbonyl group. Investigations on the properties of this new modification of the Fmoc-system, such as the solvent-dependent photochemical cleavage, and enhanced lability towards bases, are described, as well as UV-kinetic measurements of the cleavage reaction. In addition, the incorporation of the (2-nitrofluoren-9-yl)methoxycarbonyl group into two peptides, and a sequence-dependent photochemical cleavage reaction are reported.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
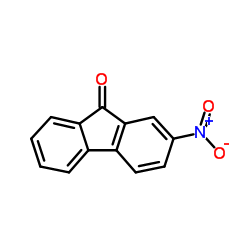 |
2-硝基芴酮
CAS:3096-52-4 |
C13H7NO3 |
相关文献:
更多...
|
Laser desorption/ionization time-of-flight mass spectrometry...
1996-07-15 [Anal. Chem. 68(14) , 2319-24, (1996)] |
|
2-Nitrofluorene and related compounds: prevalence and biolog...
1988-09-01 [Mutat. Res. 196(2) , 177-209, (1988)] |
|
Voltammetric Determination of Genotoxic Nitro Derivatives of...
[Electroanalysis 22(17-18) , 2034-2042, (2010)] |
|
Isolation, identification and bacterial mutagenicity of 2-ni...
1986-02-01 [Mutat. Res. 173(2) , 105-9, (1986)] |
|
2-Nitrofluoren-9-one: a unique mutagen formed in the photo-o...
1986-03-01 [Carcinogenesis 7(3) , 499-502, (1986)] |