Synthesis and in vitro antiviral activity of 3'-O-acyl derivatives of 5'-amino-5'-deoxythymidine: potential prodrugs for topical application.
T S Lin
文献索引:J. Pharm. Sci. 73(11) , 1568-71, (1984)
全文:HTML全文
摘要
A series of 3'-O-acyl derivatives of 5'-amino-5'-deoxythymidine (5'-NH2-TdR) (IIIa-j) was synthesized by acylation of 5'-azido-5'-deoxythymidine (I). The resulting acetoxy azides were reduced by catalytic hydrogenation to give the corresponding amines. The antiviral activity against herpes simplex virus type 1 (HSV-1) in vitro, the aqueous solubilities, and the octanol-water partition coefficients of these compounds were determined. All these derivatives have shown potency against HSV-1 virus similar to the parent compound, except the aromatic and highly branched aliphatic esters, IIIi and IIIj, which are less active. Because of their markedly improved lipophilicity, these compounds are believed to penetrate the biological membranes more easily, and thus, would be more effective for the topical treatment of cutaneous herpes virus infections.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
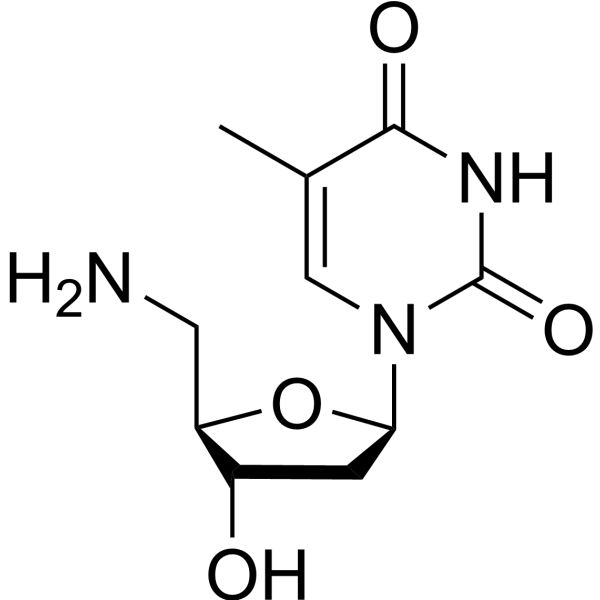 |
5-氨基-5-脱氧胸腺嘧啶脱氧核苷
CAS:25152-20-9 |
C10H15N3O4 |
|
Measurement of carbamoylating activity of nitrosoureas and i...
1986-07-15 [Biochem. Pharmacol. 35(14) , 2359-65, (1986)] |
|
Novel acyclic amide-conjugated nucleosides and their analogu...
2009-02-01 [Nucleosides Nucleotides Nucleic Acids 28 , 103-117, (2009)] |
|
Carbamoylation reactions of N-methyl-N'-aryl-N-nitrosoureas ...
1992-04-01 [Carcinogenesis 13(4) , 699-702, (1992)] |
|
Template-directed addition of nucleosides to DNA by the BfiI...
2008-07-01 [Nucleic Acids Res. 36 , 3969-3977, (2008)] |
|
Modulation of the metabolism and cytotoxicity of iododeoxyur...
1983-05-01 [Mol. Pharmacol. 23(3) , 709-16, (1983)] |