Identification, characterization and synthesis of impurities of zafirlukast.
Gilla Goverdhan, Anumula Raghupathi Reddy, Kurella Srinivas, Vurimidi Himabindu, Ghanta Mahesh Reddy
文献索引:J. Pharm. Biomed. Anal. 49(4) , 895-900, (2009)
全文:HTML全文
摘要
Zafirlukast is a drug in the treatment of pulmonary disorders such as asthma. During the process development of zafirlukast, five unknown impurities were detected at levels of below 0.10% (ranging from 0.05 to 0.15%) in reverse phase gradient high performance liquid chromatography (HPLC) method. The molecular weights were determined by LC-MS analysis. These impurities were isolated from crude samples of zafirlukast using gradient reverse phase preparative HPLC and were subsequently synthesized. Based on the spectral data, the structures of these impurities were characterized as 3-methoxy-4-(5-methoxycarbonylamino-1-methyl-1H-indol-3-ylmethyl)-benzoic acid (Impurity 1), {3-[2-methoxy-4-(toluene-2-sulfonylaminocarbonyl)-benzyl]-1-methyl-1H-indol-5-yl}-carbamic acid methyl ester (Impurity 2), {3-[2-methoxy-4-(toluene-3-sulfonylaminocarbonyl)-benzyl]-1-methyl-1H-indol-5-yl}-acetic acid cyclopentyl ester (Impurity 3), {3-[2-methoxy-4-(toluene-4-sulfonylaminocarbonyl)-benzyl]-1-methyl-1H-indol-5-yl}-acetic acid cyclopentyl ester (Impurity 4), and 4-(5-cyclopentyloxy carbonylamino-1-methyl-1H-indol-3-yl methyl)-3-methoxy-benzoic acid methyl ester (Impurity 5). The separation of the impurities by reverse phase HPLC, the confirmation of their structures by IR, MS and NMR spectral data, the mechanism of their formation and their syntheses are discussed in detail.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
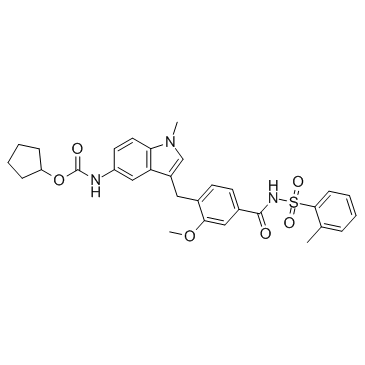 |
扎鲁司特
CAS:107753-78-6 |
C31H33N3O6S |
|
Novel strategy for biofilm inhibition by using small molecul...
2015-01-01 [Antimicrob. Agents Chemother. 59(1) , 633-41, (2014)] |
|
Rapid determination of canagliflozin in rat plasma by UHPLC-...
2015-01-01 [Talanta 132 , 29-36, (2014)] |
|
Volume-sensitive release of organic osmolytes in the human l...
2013-07-01 [Am. J. Physiol. Cell Physiol. 305(1) , C48-60, (2013)] |
|
Differential inhibitory effects of CysLT(1) receptor antagon...
2011-01-01 [PLoS ONE 6 , e22363, (2011)] |
|
Identification of novel functional inhibitors of acid sphing...
2011-01-01 [PLoS ONE 6 , e23852, (2011)] |