Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose.
V Bille, J Remacle
文献索引:Eur. J. Biochem. 160(2) , 343-8, (1986)
全文:HTML全文
摘要
Yeast alcohol dehydrogenase was successfully immobilized on tresyl-chloride-activated agarose; the optimized conditions allowed an enzyme activity recovery of over 90%. Comparison of free and immobilized enzyme properties showed an unchanged intrinsic activation energy of the reaction and a shift of optimum activity to a higher pH medium after immobilization. Comparison of the kinetic parameters for both substrates of the reaction showed that the Michaelis-Menten model could not take into consideration all the constraints induced by the immobilization on the enzyme properties but that the Theorell-Chance model was more appropriate. These results are discussed taking into consideration the factors affecting the immobilized enzyme. Finally, we discuss the possibilities of cofactor regeneration with this immobilized alcohol dehydrogenase.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
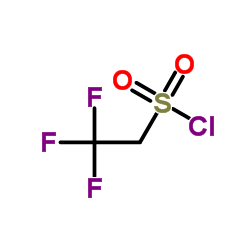 |
2,2,2-三氟乙基磺酰氯
CAS:1648-99-3 |
C2H2ClF3O2S |
|
Tresyl-based conjugation of protein antigen to lipid nanopar...
2010-01-01 [Int. J. Pharm. 401(1-2) , 87-92, (2010)] |
|
Compared stability of Sepharose-based immunoadsorbents prepa...
[J. Chromatogr. A. 584(1) , 17-22, (1992)] |
|
Multifunctional commercially pure titanium for the improveme...
2016-03-01 [Mater. Sci. Eng. C. Mater. Biol. Appl. 60 , 384-93, (2015)] |
|
Tresyl-mediated synthesis: kinetics of competing coupling an...
1999-01-01 [Bioconjug. Chem. 10(2) , 213-20, (1999)] |
|
Receptor-specific targeting with liposomes in vitro based on...
2009-03-01 [Pharm. Res. 26(3) , 529-38, (2009)] |