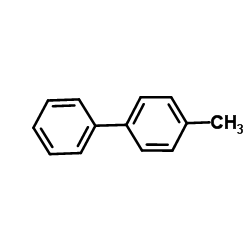
|
~92% |
|
~39% |
|
~72% |
|
~70% |
|
~71% |
|
~43% |

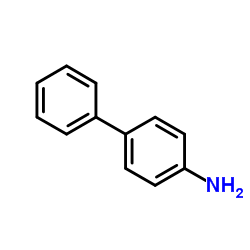
![[1,1'-Biphenyl]-4-amine,N,N-dimethyl结构式](https://image.chemsrc.com/caspic/481/1137-79-7.png)


![N,N-dimethyl-[1,1'-biphenyl]-2-amine结构式](https://image.chemsrc.com/caspic/339/6590-81-4.png)