Systemic absorption of 3H-fenticonazole after vaginal administration of 1 gram in patients.
A Novelli, E Periti, G B Massi, R Masi, T Mazzei, P Periti
文献索引:J. Chemother. 3(1) , 23-7, (1991)
全文:HTML全文
摘要
Fourteen women, five with normal cervicovaginal mucosa (Group 1), five with cervical carcinoma (Group 2) and four with relapsing vulvovaginal candidiasis (Group 3) were enrolled and completed this open clinical trial. Each subject received a single dose of 1.82 +/- 0.3 g on average of vaginal paste (for ovules) containing about 1000 mg of 3H-fenticonazole nitrate (266 microCi). Twelve hours after vaginal administration, the paste was removed by vaginal washing. Blood, urine and stool samples were collected at specified time intervals for five days. Plasma, urine, stools and all used material in contact with the paste were assayed for radioactivity. No measurable levels of radioactivity were detected in plasma of subjects of Groups 1 and 3 while in 4 of the 5 subjects with cervical carcinoma (Group 2) fenticonazole was detected during the 24 h after administration with a peak level at about 8 hours. For a period of 5 days, 0.4-1.5% of the dose on average was recovered from urine, and 0.18-0.32% from feces. Based on the excretion data, the extent of vaginal absorption of fenticonazole nitrate in women with vulvovaginal candidiasis was 1.81 +/- 0.57% of the dose, while in women with normal cervicovaginal mucosa it accounted for 0.58 +/- 0.28% of the administered dose. In patients with cervical carcinoma, absorption was 1.12 +/- 0.53%. The maximum amount absorbed corresponds to an exposure of about 0.4 mg/kg of fenticonazole nitrate (for a subject weighing 50 kg). Consequently, the vaginal administration of one ovule containing 1000 mg of fenticonazole nitrate seems to be devoid of risk for patients.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
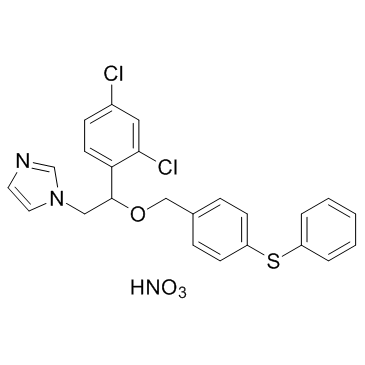 |
硝酸芬替康唑
CAS:73151-29-8 |
C24H21Cl2N3O4S |
|
In vitro activity of cloconazole, sulconazole, butoconazole,...
1992-04-01 [Mycopathologia 118(1) , 15-21, (1992)] |
|
Fenticonazole activity measured by the methods of the Europe...
2009-05-01 [Antimicrob. Agents Chemother. 53 , 2181-4, (2009)] |
|
Single dose therapy of vaginal candidiasis: a comparative tr...
1990-01-01 [Curr. Med. Res. Opin. 12(2) , 114-20, (1990)] |
|
Chiral discrimination by HPLC and CE and antifungal activity...
2002-05-15 [Chirality 14(5) , 449-54, (2002)] |
|
The development and validation of a high performance liquid ...
1995-01-01 [Rapid Commun. Mass Spectrom. 9(14) , 1452-6, (1995)] |