Single dose therapy of vaginal candidiasis: a comparative trial of fenticonazole vaginal ovules versus clotrimazole vaginal tablets.
A G Lawrence, E T Houang, E Hiscock, M B Wells, E Colli, M Scatigna
文献索引:Curr. Med. Res. Opin. 12(2) , 114-20, (1990)
全文:HTML全文
摘要
An open, randomized comparative clinical trial was performed in 153 patients suffering from symptomatic vaginal candidiasis confirmed by mycological tests. Patients were allocated at random into two groups: the first group (consisting of 75 subjects) was treated with a single vaginal ovule of fenticonazole (600 mg) and the second group (consisting of 78 subjects) was treated with a single vaginal tablet of clotrimazole (500 mg). Therapeutic efficacy was assessed by microbiological and clinical criteria 7 days and 1 month (when possible) after the single dose treatment. At the first follow-up visit, complete disappearance of the signs and symptoms or a highly significant reduction of their intensity was observed in both treatment groups. No significant difference was evident between the two drugs. At 7 days, the mycological tests gave negative results in 92% of the patients in the fenticonazole group and in 88.5% of the patients in the clotrimazole group. The difference between the two treatment groups was again not statistically significant. The second follow-up visit was performed in 55 (73.3%) patients of the fenticonazole group and in 52 (66.7%) patients of the clotrimazole group. The results indicate that 83.6% of patients in the fenticonazole group and 69.2% of patients in the clotrimazole group were still disease free at the time of this visit. Both drugs were well tolerated. Mild, local and short lasting side-effects were reported in only 5 cases of the group treated with fenticonazole.(ABSTRACT TRUNCATED AT 250 WORDS)
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
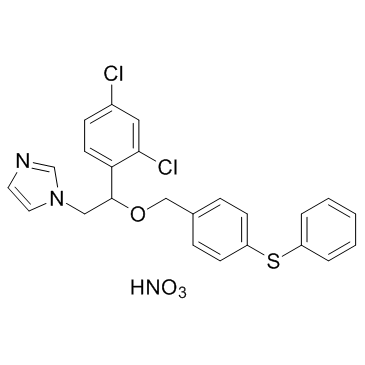 |
硝酸芬替康唑
CAS:73151-29-8 |
C24H21Cl2N3O4S |
|
In vitro activity of cloconazole, sulconazole, butoconazole,...
1992-04-01 [Mycopathologia 118(1) , 15-21, (1992)] |
|
Fenticonazole activity measured by the methods of the Europe...
2009-05-01 [Antimicrob. Agents Chemother. 53 , 2181-4, (2009)] |
|
Chiral discrimination by HPLC and CE and antifungal activity...
2002-05-15 [Chirality 14(5) , 449-54, (2002)] |
|
The development and validation of a high performance liquid ...
1995-01-01 [Rapid Commun. Mass Spectrom. 9(14) , 1452-6, (1995)] |
|
Blood partition and protein binding of a new reversible prot...
1998-09-01 [Biopharm. Drug Dispos. 19(6) , 413-5, (1998)] |