Carbohydrate Research
2004-01-22
An efficient chemoenzymatic route to methyl 4-O-benzyl-2,3-anhydro-beta-D-lyxopyranoside from methyl beta-D-xylopyranoside.
Mária Mastihubová, Vladimír Mastihuba, Peter Biely
文献索引:Carbohydr. Res. 339(2) , 425-8, (2004)
全文:HTML全文
摘要
Methyl 4-O-benzyl-2,3-anhydro-beta-D-lyxopyranoside, an intermediate for the preparation of methyl beta-D-xylopyranoside derivatives modified at C-2, was obtained in five steps in 58% yield. The synthetic sequence starts from methyl beta-D-xylopyranoside through two main steps involving regioselective enzymatic acetylation and deacetylation catalyzed by lipase PS.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
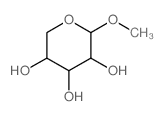 |
甲基 β-吡喃木糖苷
CAS:612-05-5 |
C6H12O5 |
相关文献:
更多...
|
Effects of inhibition of proteoglycan synthesis on the diffe...
1987-08-01 [J. Cell Biol. 105(2) , 1013-21, (1987)] |
|
Tetraisopropyldisiloxane-1,3-diyl as a versatile protecting ...
2012-05-15 [Carbohydr. Res. 353 , 92-5, (2012)] |
|
Transacetylations to carbohydrates catalyzed by acetylxylan ...
2003-10-13 [Biochim. Biophys. Acta 1623 , 62-71, (2003)] |
|
Synthesis of acylated methyl beta-D-xylopyranosides and thei...
1997-07-11 [Carbohydr. Res. 302(1-2) , 13-8, (1997)] |
|
Determination of sugar structures in solution from residual ...
2004-10-13 [J. Am. Chem. Soc. 126(40) , 13100-10, (2004)] |