Homogeneous assays for riboflavin mediated by the interaction between enzyme-biotin and avidin-riboflavin conjugates.
M J Kim, H J Kim, J M Kim, B Kim, S H Han, G S Cha
文献索引:Anal. Biochem. 231 , 400-406, (1995)
全文:HTML全文
摘要
Homogeneous-type enzyme-linked competitive binding assays utilizing the synthetic enzyme-biotin and avidin-riboflavin conjugates are developed for the detection of riboflavin as well as its binder protein. The activity of the enzyme-biotin conjugate is inhibited in the presence of the avidin-riboflavin conjugate, and the observed inhibition is reversed in an amount dependent on the concentration of riboflavin binding protein (RBP) added. Upon additions of free riboflavin to the mixture, activity is reinhibited in an amount proportional to the riboflavin concentration. Three different enzymes are examined as the labels: glucose-6-phosphate dehydrogenase, adenosine deaminase, and alkaline phosphatase. The catalytic activity of these enzymes, when conjugated with biotin, is shown to be inhibited to a significant degree (> 90%) by the binding of the avidin-riboflavin conjugate, and reversed upon additions of RBP.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
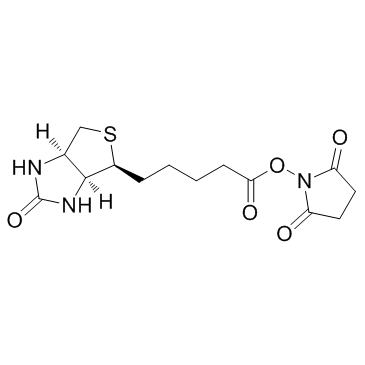 |
(+)生物素-N-琥珀酰亚胺基酯
CAS:35013-72-0 |
C14H19N3O5S |
|
Rapid fluorescent detection of Escherichia coli K88 based on...
2014-07-01 [J. Fluoresc. 24(4) , 1159-68, (2014)] |
|
Xepac protein and IP3/Ca2+ pathway implication during Xenopu...
2015-02-01 [Zygote 23(1) , 99-110, (2015)] |
|
The use of the avidin-biotin complex as a tool in molecular ...
1980-01-01 [Methods Biochem. Anal. New York, NY 26 , 1-45, (1980)] |
|
K. Hoffmann et al.
[J. Am. Chem. Soc. 100 , 3585, (1978)] |
|
On-resin biotinylation of chemically synthesized proteins fo...
1988-05-01 [Anal. Biochem. 170 , 502, (1988)] |